31 Spectra (Graded Reading)
Janice Hester
There are two types of light that we can observe from any object. The first is reflected light. Most of the light we see is created by the Sun, a light bulb, a screen, or another light source and then reflects off of the objects around us before entering our eyes.
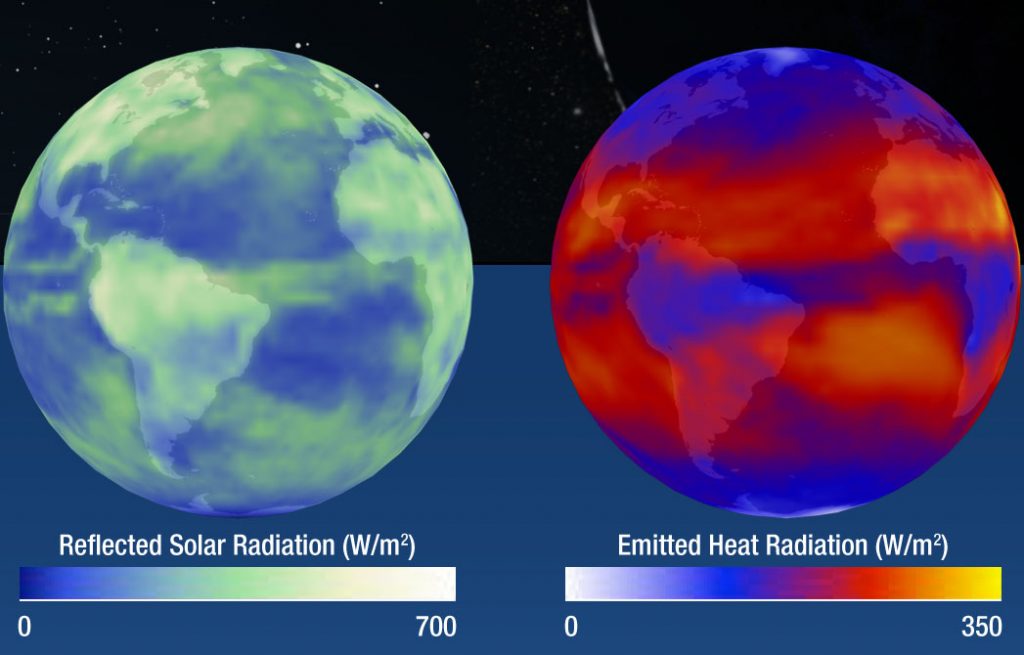
The other type of light is light that is radiated by an object itself. In our solar system, the Sun is the most obvious example of an object radiating its own light, but it isn’t the only one. Light is created whenever a charged particle accelerates. For example, all dense objects emit thermal radiation that is created by the random thermal motions of the object’s atoms and molecules. People and animals, with body temperatures around 310 K, radiate at wavelengths around 9 microns. You can see this light with an infrared (IR) camera. The Earth, with an average surface temperature of 288 K also emits thermal radiation in the IR. The image below shows sunlight reflected from the Earth’s atmosphere and surface (visible light) and thermal emission from the Earth’s atmosphere and surface (IR light).
The light reflected by, absorbed by, and emitted by planets, stars, nebulae, galaxies, etc. is the primary way that we learn about these objects. Within our own solar system, missions have sampled the atmospheres and surfaces of other worlds, analyzed meteorites and sample returns, and recorded martian earthquakes, but we always start by observing light.
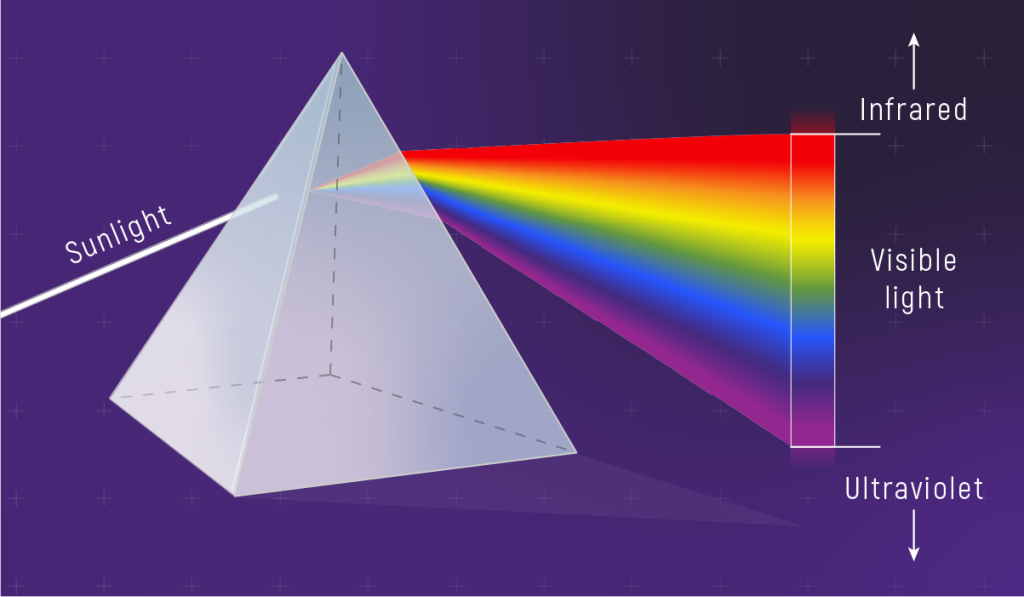
To learn the most from the light coming from an object, we spread the light out into a spectrum. The different wavelengths of light are separated and the intensity of light in each wavelength is measured. Splitting light into a spectrum (separating it by wavelength) can be done with a prism or a diffraction grating. The prism works on optics principles, the bending of light rays of different wavelengths as they pass into and out of the glass prism. The grating takes advantage of the wave properties of light; different wavelengths (colors) of light passing through the narrow lines in the grating interfere constructively and destructively at different angles. Both technologies separate light into its different wavelengths.
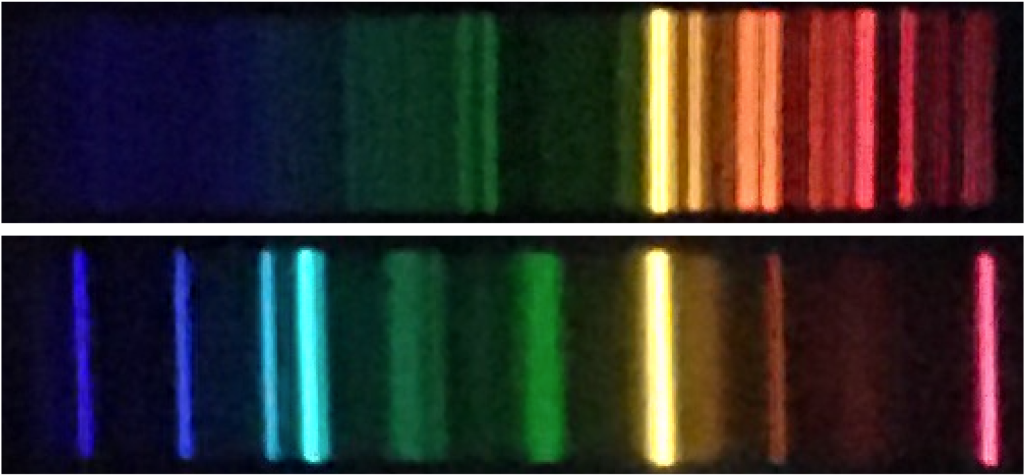
The spectrum of a object can be represented in two ways. The first is a colored band that shows what you would see if you looked at the spectrum. The images below are example spectra taken through a spectral viewer; these are the type of spectra you might observe in an introductory astronomy lab. Notice that not all wavelengths of light are present in these spectra. For example, both of the sources used to create these spectra emit strongly (emit a lot of light) around a particular yellow wavelength, but many wavelengths of blue light are missing.
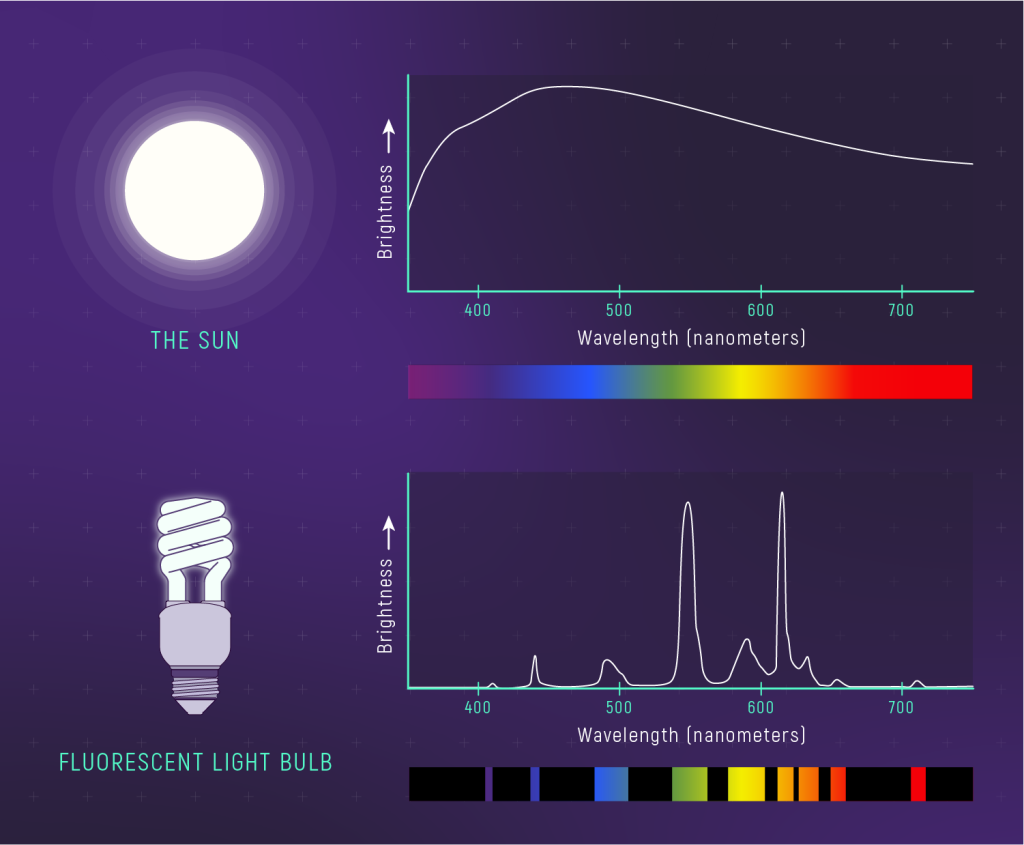
The other way to represent a spectrum is as a graph of brightness (really intensity) versus wavelength. The figure below shows a picture of the spectrum (what you would observe) alongside a graph of the spectrum for two different light sources. The spectrum of sunlight is continuous; it contains all visible wavelengths of light; the graph of brightness versus wavelength is smooth and unbroken. A compact fluorescent light bulb doesn’t emit all wavelengths of light. The spectrum is made up of broad bars of color, which show up as peaks in the spectrum’s graph.