1.14: Phase Transitions – Melting, Boiling, and Subliming
Learning Objectives
- Describe what happens during a phase change.
- Understand the terms used to describe each phase change.
- Describe kinetic molecular theory and how it is related to the behavior of gases.
Phase Transitions (Phase Changes)
Substances can change their physical phase, or state, often because of a temperature change. This process is called a phase transition or phase change. At lower temperatures, most substances are solid; as the temperature increases, they become liquid; and at higher temperatures still, they become gaseous. Water provides a familiar example of these phases: the solid phase is ice, the water we drink is in the liquid phase, and the gas phase is water vapor (or steam).
Phase transitions are physical changes: the substance changes its physical state but is still the same substance (e.g. liquid water and ice are both water, just in different physical states). As we will explore later in the course, a chemical reaction involves a chemical change, where a substance is transformed into an entirely different substance.
Melting and Freezing
The process of a solid becoming a liquid is called melting (an older term that you may see sometimes is fusion). The opposite process, a liquid becoming a solid, is called solidification or freezing. For any pure substance, the temperature at which melting occurs—known as the melting point—is a characteristic of that substance. It requires energy for a solid to melt into a liquid. Every pure substance has a certain amount of energy it needs to change from a solid to a liquid.
As melting is occurring, energy goes exclusively to changing the phase of a substance; it does not go into changing the temperature of the substance. Hence melting (or any phase transition) is an isothermal (iso: same; thermal: heat) process because a substance stays at the same temperature as it melts (or as it undergoes any phase change). Only when all of a substance is melted does additional energy go to changing its temperature.
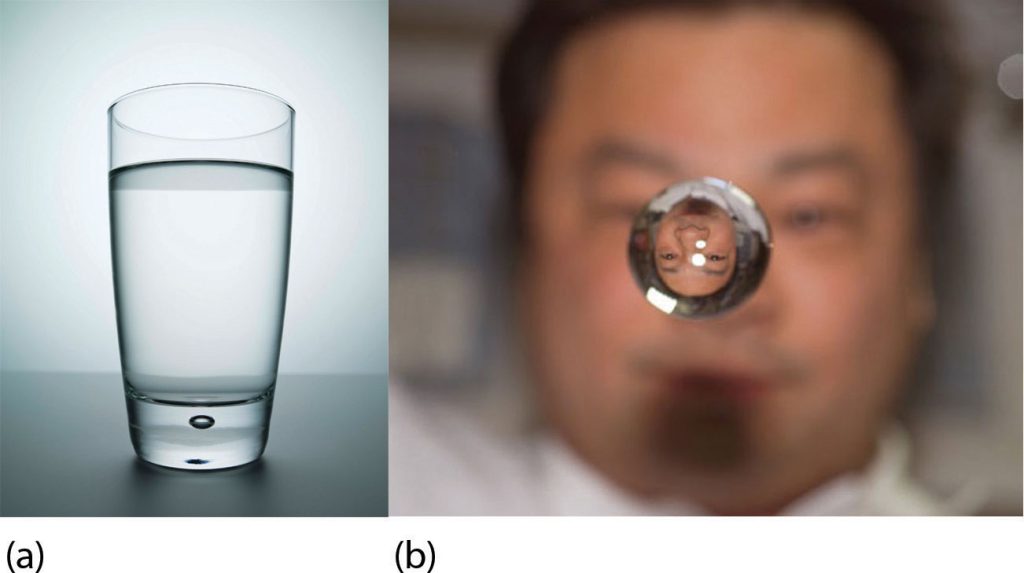
What happens when a solid becomes a liquid? In a solid, individual particles are stuck in place because the attractive forces between the particles (“intermolecular forces”) cannot be overcome by the energy of the particles. When more energy is supplied (e.g., by raising the temperature), there comes a point at which the particles have enough energy to move around, but not enough energy to separate. This is the liquid phase: particles are still in contact, but are able to move around each other. This explains why liquids can assume the shape of their containers: the particles move around and, under the influence of gravity, fill the lowest volume possible (unless the liquid is in a zero-gravity environment—see Figure 1.14.1).
Vaporization, Condensation, and Kinetic Molecular Theory
The phase change between a liquid and a gas has some similarities to the phase change between a solid and a liquid. At a certain temperature, the particles in a liquid have enough energy to become a gas. The process of a liquid becoming a gas is called boiling (or vaporization), while the process of a gas becoming a liquid is called condensation. However, unlike the solid/liquid conversion process, the liquid/gas conversion process is noticeably affected by the surrounding pressure on the liquid because gases are strongly affected by pressure. This means that the temperature at which a liquid becomes a gas, the boiling point, can change with surrounding pressure. Therefore, we define the normal boiling point as the temperature at which a liquid changes to a gas when the surrounding pressure is exactly 1 atm, or 760 torr. (The abbreviations “atm” and “torr” are two of the units used when we make measurements of pressure.) Unless otherwise specified, it is assumed that a boiling point is for 1 atm of pressure.
Like the solid/liquid phase change, the liquid/gas phase change involves energy, and the energy in boiling goes exclusively to changing the phase of a substance; it does not go into changing the temperature of a substance. So boiling is also an isothermal process. Only when all of a substance has boiled does any additional energy go to changing its temperature.
What happens when a liquid becomes a gas? The behavior of molecules when they are in the gas phase is described by kinetic molecular theory. We have already established that a liquid is composed of particles in contact with each other. When a liquid becomes a gas, the particles separate from each other, with each particle going its own way in space. Kinetic molecular theory is based on three ideas: first, that the volume of a single gas molecule or particle is tiny compared to the volume of the container; second, that the gas particles are in constant motion; and third, at a particular temperature all the gas particles have the same kinetic energy (energy of motion). These ideas help explain how gases tend to fill their containers. Indeed, in the gas phase most of the volume is empty space; only about 1/1,000th of the volume is actually taken up by matter (Figure 1.14.2). It is this property of gases that explains why they can be compressed.
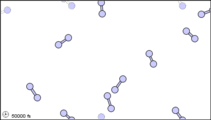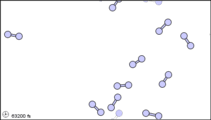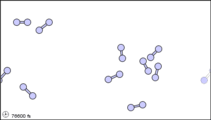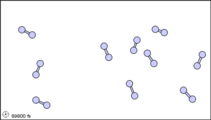

Figure 1.14.2: Sub-microscopic view of the diatomic molecules of the element bromine (a) in the gaseous state (above 58°C); (b) in liquid form (between -7.2 and 58.8°C); and (c) in solid form (below -7.2°C). As a solid, the molecules are fixed, but fluctuate. As a liquid, the molecules are in contact but are also able to move around each other. As a gas, most of the volume is actually empty space. The particles are not to scale; in reality, the dots representing the particles would be about 1/100th of the size depicted.
Sublimation and Deposition
Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. The solid-to-gas change is called sublimation, while the reverse process is called deposition. Sublimation is isothermal, like the other phase changes, and there is a measurable energy change during sublimation.
There are several common examples of sublimation. A well-known product, dry ice, is actually solid CO2. Dry ice is dry because it sublimes, with the solid bypassing the liquid phase and going straight to the gas phase. The sublimation occurs at temperature of −77°C, so it must be handled with caution. If you have ever noticed that ice cubes in a freezer tend to get smaller over time, it is because the solid water is very slowly subliming. “Freezer burn” isn’t actually a burn; it occurs when certain foods, such as meats, slowly lose solid water content because of sublimation. The food is still good, but looks unappetizing. Reducing the temperature of a freezer will slow the sublimation of solid water.
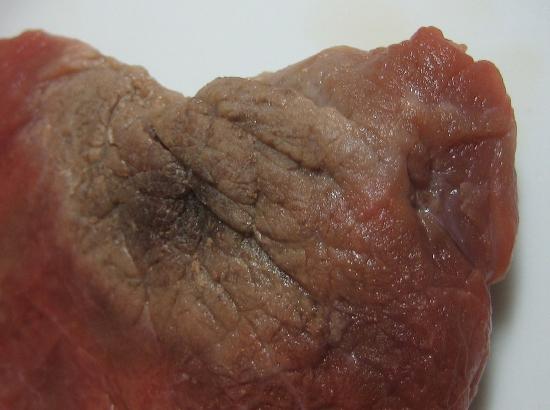
Chemical equations can be used to represent a phase change. In such cases, it is crucial to use phase labels on the substances. For example, the chemical equation for the melting of ice to make liquid water is as follows:
H2O(s) → H2O(ℓ)
No chemical change is taking place; however, a physical change is taking place.
Key Takeaways
- Phase changes can occur between any two phases of matter (solid, liquid, and gas).
- All phase changes occur with a simultaneous change in energy.
- All phase changes are isothermal, meaning that the temperature of the substance stays the same until the phase change is complete.
Supplemental Video Support

