3.6: Naming Covalent (Molecular) Compounds
Learning Objective
- Determine the name of a simple covalent compound from its chemical formula.
Covalent compounds, also known as molecular compounds, are inorganic compounds that take the form of discrete molecules. Examples include such familiar substances as water (H2O) and carbon dioxide (CO2). Covalent compounds contain two or more nonmetals. These compounds are very different from ionic compounds like sodium chloride (NaCl); as we’ve seen, ionic compounds are formed when metal atoms lose one or more of their electrons to nonmetal atoms and the resulting cations and anions are electrostatically attracted to each other.
So what holds the atoms of a molecule together? Rather than forming ions, the atoms of a molecule share their electrons in such a way that a bond forms between a pair of atoms. In a carbon dioxide molecule, there are two of these bonds, each occurring between the carbon atom and one of the two oxygen atoms.
Larger molecules can have many, many bonds that serve to keep the molecule together. In a large sample of a given covalent compound, all of the individual molecules are identical.
Naming Binary Covalent Compounds
Recall that a molecular formula shows the number of atoms of each element that a molecule contains. A molecule of water contains two hydrogen atoms and one oxygen atom, so its formula is H2O. A molecule of octane, which is a component of gasoline, contains 8 atoms of carbon and 18 atoms of hydrogen. The molecular formula of octane is C8H18.

Naming binary (two element) covalent compounds is similar to naming simple ionic compounds. The first element in the formula is simply listed using the name of the element. The second element is named by taking the stem of the element name and adding the suffix –ide. A system of numerical prefixes is used to specify the number of atoms in a molecule. Table 3.6.1 lists these numerical prefixes.
Table 3.6.1: Numerical Prefixes for Naming Binary Covalent Compounds
| Number of Atoms in Compound | Prefix on the Name of the Element |
| 1 | mono-* |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
| 8 | octa- |
| 9 | nona- |
| 10 | deca- |
| *This prefix is not used for the first element’s name. | |
Note
- Generally, the less electronegative element is written first in the formula, though there are a few exceptions. Carbon is always first in a formula and hydrogen is after nitrogen in a formula such as NH3. The order of common nonmetals in binary compound formulas is C, P, N, H, S, I, Br, Cl, O, F.
- The a or o at the end of a prefix is usually dropped from the name when the name of the element begins with a vowel. As an example, four oxygen atoms, is tetroxide instead of tetraoxide.
- The prefix is “mono” is not added to the first element’s name if there is only one atom of the first element in a molecule.
Some examples of molecular compounds are listed in Table 3.6.2.
Table 3.6.2: Examples of Molecular Compounds
| Formula | Name |
| NO | nitrogen monoxide |
| N2O | dinitrogen monoxide |
| S2Cl2 | disulfur dichloride |
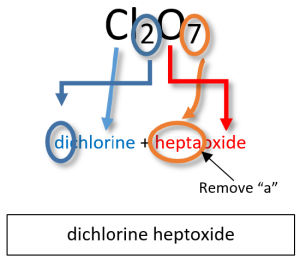
| Cl2O7 | dichlorine heptoxide |
Notice that the mono- prefix is not used with the nitrogen in the first compound, but is used with the oxygen in both of the first two examples. The example S2Cl2 illustrates that the formulas for molecular compounds are not reduced to their lowest ratios as they are for ionic compounds. The ‘o’ of the mono- and the ‘a’ of hepta- are dropped from the name when paired with oxide.
Exercise 3.6.1
Write the name for each compound.
a. CF4
b. SeCl2
c. SO3
Answers
a. carbon tetrafluoride
b. selenium dichloride
c. sulfur trioxide
Simple Covalent Compounds With Common Names
In chemical nomenclature, a systematic name is one that follows a set of rules for naming compounds. A common name is one that does not follow any rules, but has been in use for so long that it is a generally accepted name for a compound. For some simple covalent compounds, we use common names rather than systematic names. We have already encountered these compounds, but we list them here explicitly:
- H2O: water
- NH3: ammonia
- CH4: methane
- H2O2: hydrogen peroxide
Methane is the simplest organic compound. Organic compounds are compounds with carbon atoms and are named by a separate nomenclature system.
Some Compounds Have Both Covalent and Ionic Bonds
If you recall the introduction of polyatomic ions, you will remember that the bonds that hold the polyatomic ions together are covalent bonds. Once the polyatomic ion is constructed with covalent bonds, it reacts with other substances as an ion. The bond between a polyatomic ion and another ion will be ionic. An example of this type of situation is in the compound sodium nitrate. Sodium nitrate is composed of a sodium ion and a nitrate ion. The nitrate ion is held together by covalent bonds and the nitrate ion is attached to the sodium ion by an ionic bond.
Key Takeaways
- A covalent compound is usually composed of two or more nonmetal elements.
- Covalent compounds are named with the first element first and then the second element by using the stem of the element name plus the suffix -ide. Numerical prefixes are used to specify the number of atoms in a molecule.
Supplemental Video Support
Contributions & Attributions
- Marisa Alviar-Agnew (Sacramento City College)
- Henry Agnew (UC Davis)

