3.7: Naming Acids
A “spot test” (a quick, informal, qualitative test) for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not. Then the sample is added to a mixture of nitric acid and hydrochloric acid. Gold will only dissolve in this mixture. The term “acid test” arose from the California gold rush in the late 1840’s when this combination was used to test for the presence of real gold. It has since come to mean, “tested and approved” in a number of fields.
Acids
An acid can be defined in several ways. We’ll explore these definitions later in the course, but for now the most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions (H+) when dissolved in water. When a substance dissolves in water, we write (aq), meaning aqueous, after its formula. Thus, for naming acids, the convention we will follow is to write (aq) after the formula.

This is a different type of compound than the others we have seen so far. Acids are molecular, which means that in their pure state they are individual molecules and do not adopt the extended three-dimensional structures of ionic compounds like NaCl. However, when these molecules are dissolved in water, the chemical bond between the hydrogen atom and the rest of the molecule breaks, leaving a positively-charged hydrogen ion and an anion. This can be symbolized in a chemical equation:
HCl ⇒ H+ + Cl–
Since all acids contain hydrogen, the name of an acid is based on the anion that goes with it. These anions can either be monatomic or polyatomic.
Naming Binary Acids
A binary acid is an acid that consists of hydrogen and one other nonmetal element. The most common binary acids contain a halogen in the form of a halide ion (a halide ion is the anion of a halogen). The acid name begins with the prefix hydro-, followed by the base name of the anion, followed by the suffix -ic.
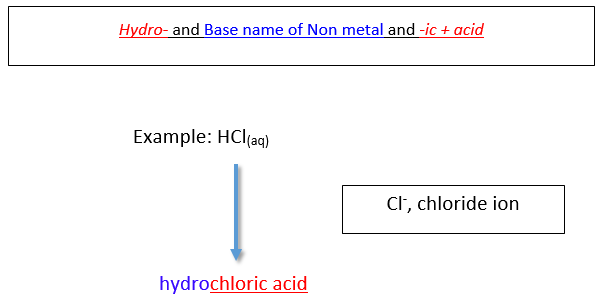
Naming Acids Based on Polyatomic Ions
When an acid is derived from a polyatomic ion, it consists of hydrogen and a polyatomic ion. (Because the polyatomic ions typically involved in acids contain oxygen, these acids are also often called oxyacids. An oxyacid is an acid that consists of hydrogen, oxygen, and a third element that is usually a nonmetal.) These types of acids are named according to the ending of the name of the polyatomic ion. There are two cases:
a. Acids based on polyatomic ions that end in -ite
The name of the acid is the root of the anion followed by the suffix -ous. There is no prefix.
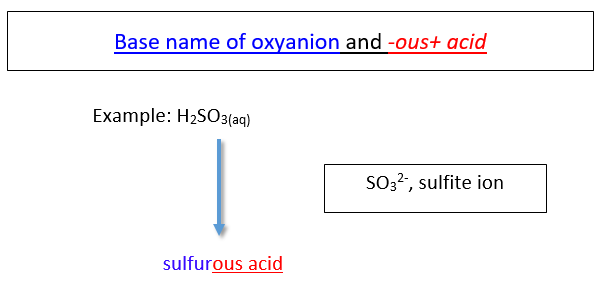
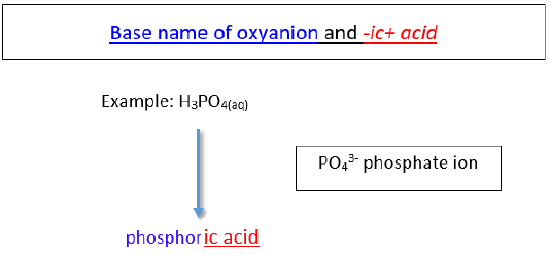
b. Acids based on polyatomic ions that end in -ate
The name of the acid is the root of the anion followed by the suffix -ic. There is no prefix.
Note
The base name for sulfur containing oxyacid is sulfur- instead of just sulf-. The same is true for a phosphorus containing oxyacid. The base name is phosphor- instead of simply phosph-.
Writing Formulas for Acids
Remember that based on the definition of acids as substances that produce hydrogen ions when dissolved in water, we will include (aq) after the formula for an acid. This practice distinguishes acids from other molecular compounds. Like other compounds that we have studied, acids are electrically neutral. Therefore, the charge of the anion part of the formula must be exactly balanced out by the H+ ions. Another way to think about writing the correct formula is to utilize the crisscross method, shown below for sulfuric acid.
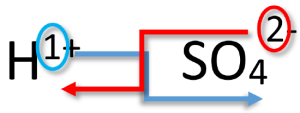
Key Takeaways
- Acids are molecular compounds that release hydrogen ions.
- A binary acid consists of hydrogen and one other element.
- Oxyacids contain hydrogen, oxygen, and one other element.
- The name of the acid is based on the anion attached to the hydrogen.
Supplemental Video Support
Contributions & Attributions
- Marisa Alviar-Agnew (Sacramento City College)
- Henry Agnew (UC Davis)

