6.8: Representing Valence Electrons with Dots
Learning Objective
- Draw a Lewis electron dot diagram for an atom or a monatomic ion.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. A simple way of representing those valence electrons is useful to help us understand how compounds form. A Lewis electron dot diagram or Lewis symbol (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom. Valence electrons are represented as dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (The order in which the positions are used does not matter.) For example, the Lewis electron dot diagram for hydrogen is simply:
![]()
Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:
![]()
The electron dot diagram for helium, with two valence electrons, is as follows:
![]()
For elements with more than one valence electron, we place one electron on each side of the symbol before we can start pairing them. The next atom, lithium, has an electron configuration of 1s22s1, so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used:
![]()
Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of helium:
![]()
The next atom is boron. Its valence electron shell is 2s22p1, so it has three valence electrons. Again, it does not matter on which sides of the symbol the electron dots are positioned:
![]()
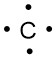
For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell:
Nitrogen has five valence electrons, so we place one on each side around the symbol before forming a pair:
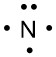
The six valence electrons of oxygen are represented in the Lewis symbol shown below. Remember that each side has no more than two electrons:

Fluorine and neon have seven and eight dots, respectively:

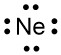
With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its highest-numbered shell, the n = 3 shell. By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol.
Example 6.8.1
What is the Lewis electron dot diagram for each element?
a. aluminum
b. selenium
Solution
a. The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum:
![]()
b. The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows:

Exercise 6.8.1
What is the Lewis electron dot diagram for each element?
a. phosphorus
b. argon
Answer
a. 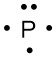
b. 
Key Takeaways
- Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
- Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom.
Supplemental Video Support
Contributions & Attributions
- Marisa Alviar-Agnew (Sacramento City College)
- Henry Agnew (UC Davis)

