6.13: Electronegativity and Polarity – Why Oil and Water Don’t Mix
Learning Objectives
- Explain how polar compounds differ from nonpolar compounds.
- Determine if a molecule is polar or nonpolar.
- Given a pair of compounds, predict which would have a higher melting or boiling point.
Bond Polarity
The ability of an atom in a molecule to attract shared electrons is called its electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom, and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom (electron transfer will occur), and the bond will be ionic. This is why metals (low electronegativities) bonded with nonmetals (high electronegativities) typically produce ionic compounds.
A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. How do we judge the degree of polarity? Scientists have devised a scale called electronegativity, a scale for judging how much atoms of any element attract electrons. Electronegativity is a unitless number; the higher the number, the more an atom attracts electrons. The most electronegative element is fluorine. Generally, electronegativity increases as you go from left to right across a period, and decreases as you go from top to bottom in a group. A common scale for electronegativity and the periodic trends for electronegativity are shown in Figure 6.13.1:
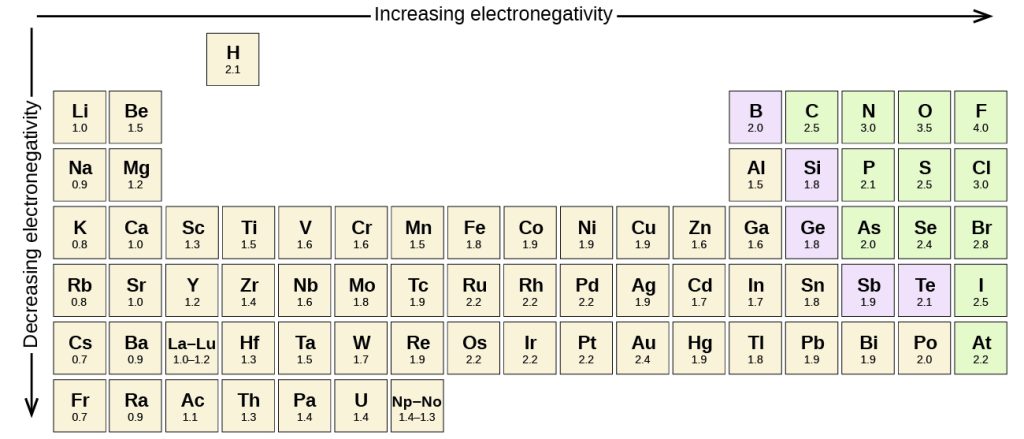
The polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized in Table 6.13.1:
Table 6.13.1: Electronegativity Difference and Bond Type
| Electronegativity Difference | Bond Type |
| 0 – 0.4 | pure covalent |
| 0.5 – 2.0 | polar covalent |
| > 2.0 | likely ionic |
Nonpolar Covalent Bonds
A bond in which the electronegativity difference is less than 1.9 is considered to be mostly covalent in character. However, at this point we need to distinguish between two general types of covalent bonds. A nonpolar covalent bond (shown in Figure 6.13.2) is a covalent bond in which the bonding electrons are shared equally between the two atoms. In a nonpolar covalent bond, the distribution of electrical charge is balanced between the two atoms.
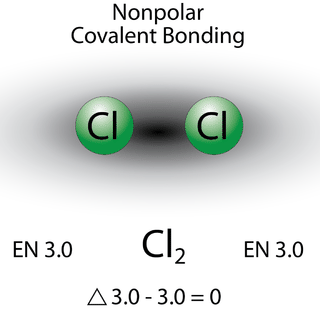
The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density surrounding the Cl2 molecule is symmetrical. Also note that molecules in which the electronegativity difference is very small (<0.5) are also considered nonpolar covalent. An example would be a bond between chlorine and bromine (ΔEN = 3.0 − 2.8 = 0.2).
Polar Covalent Bonds
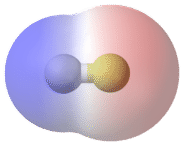
A bond in which the electronegativity difference between the atoms is between 0.5 and 2.0 is called a polar covalent bond. A polar covalent bond (Figure 6.13.3) is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. In a polar covalent bond, sometimes simply called a polar bond, the distribution of electrons around the molecule is no longer symmetrical.
An easy way to illustrate the uneven electron distribution in a polar covalent bond is to use the Greek letter delta (δ). The more electronegative element has a partial negative charge, while the less electronegative element has a partial positive charge (Figure 6.13.4):
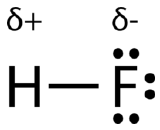
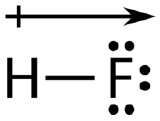
The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser electronegativity acquires a partial positive charge. The delta symbol is used to indicate that the quantity of charge is less than one. A crossed arrow called a dipole can also be used to indicate the direction of greater electron density. The arrow points toward the more electronegative element, as shown in Figure 6.13.5:
Example 6.13.1: Bond Polarity
Classify each of these bonds as polar covalent, nonpolar covalent, or ionic.
a. C-H
b. O-H
Solution
a. For the C–H bond, the difference in the electronegativities is 2.5 − 2.1 = 0.4. Thus we predict that this bond will be nonpolar covalent.
b. For the O–H bond, the difference in electronegativities is 3.5 − 2.1 = 1.4, so we predict that this bond will be polar covalent.
Exercise 6.13.1
What is the polarity of each bond?
a. Rb-F
b. P-Cl
Answer
a. ‘Likely Ionic’
b. Polar covalent
Molecular Polarity
To determine if a molecule is polar or nonpolar, it is generally useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical—bonded to the same element with no unshared pairs of electrons. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. This works pretty well, as long as you can visualize the molecular geometry. That’s the hard part. To know how the bonds are oriented in space, you have to have a strong grasp of Lewis structures and VSEPR theory. Assuming that you do, you can look at the structure of each one and decide if it is polar or not, whether or not you know the individual atom’s electronegativity. This is because you know that all bonds between dissimilar elements are polar, and in these particular examples, it doesn’t matter which direction the dipole moment vectors are pointing (out or in).
A polar molecule is a molecule in which the electrons are distributed unevenly: one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole (Figure 6.13.6).

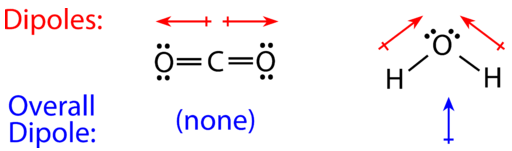
For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. Dipoles are mathematical quantities called vectors that have magnitude (which depends on the difference in electron distribution and the distance between the ends of the dipole) and direction. They can be combined to create new dipoles, although sometimes they cancel each other out and no new net dipole is created. The figure below shows a comparison between carbon dioxide and water. Carbon dioxide (CO2) is a linear molecule. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the C atom to each O atom. However, since the dipoles are of equal magnitude and pointing in opposite directions, they cancel out and the overall molecular polarity of CO2 is zero.
Water is a bent molecule because of the two lone pairs on the central oxygen atom. The individual dipoles point from the H atoms toward the O atom. Because of the shape, the dipoles do not cancel each other out and the result is an overall dipole, shown in blue in Figure 6.13.7. Thus, the water molecule is polar.
Some other molecules are shown in the figure below. Notice that a tetrahedral molecule such as CH4 is nonpolar. However, if one of the peripheral H atoms is replaced with another atom that has a different electronegativity, the molecule becomes polar. A trigonal planar molecule (BF3) may be nonpolar if all three peripheral atoms are the same, but a trigonal pyramidal molecule (NH3) is polar.
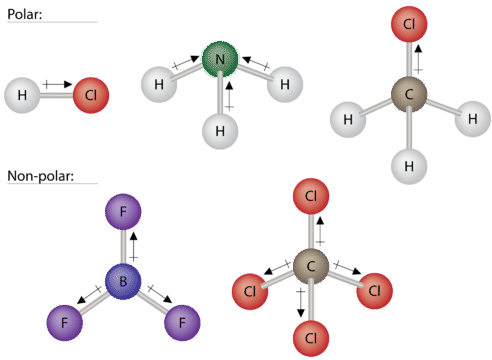
To summarize, to be polar, a molecule must:
- Contain at least one polar covalent bond.
- Have a molecular structure such that the sum of the vectors of each bond dipole moment do not cancel.
Steps to Identify Polar Molecules
- Draw the Lewis structure.
- Figure out the geometry (using VSEPR theory).
- Visualize or draw the geometry.
- Find the net dipole moment (you don’t have to actually do calculations if you can visualize it).
- If the net dipole moment is zero, it is non-polar. Otherwise, it is polar.
Properties of Polar Molecules
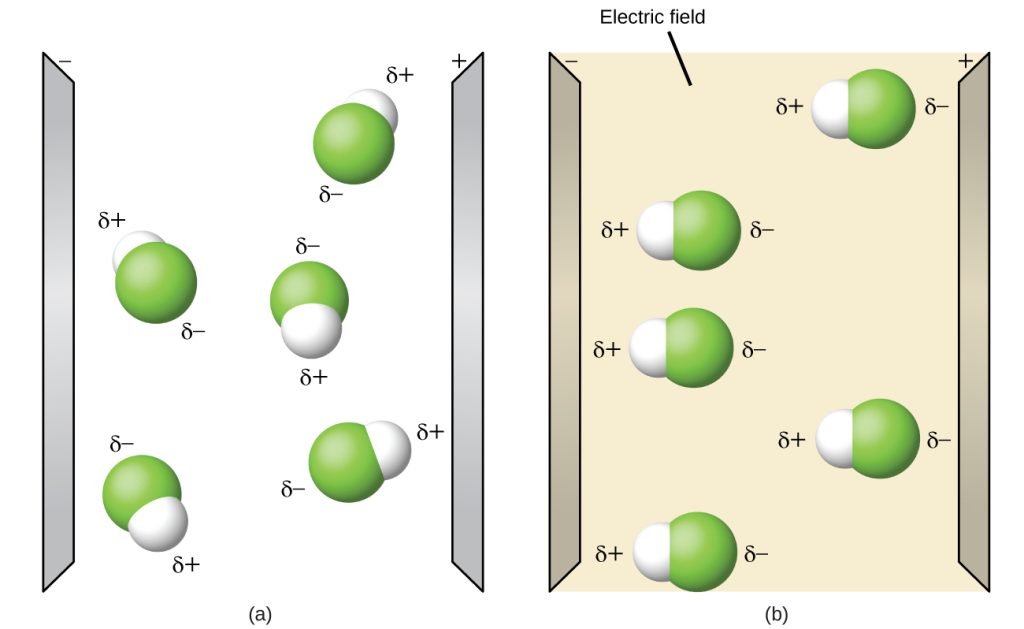
Polar molecules tend to align when placed in an electric field with the positive end of the molecule oriented toward the negative plate and the negative end toward the positive plate (Figure 6.13.9). We can use an electrically charged object to attract polar molecules, but nonpolar molecules are not attracted. Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances.
While molecules can be described as “polar covalent” or “ionic”, it must be noted that this is often a relative term, with one molecule simply being more polar or less polar than another. However, the following properties are typical of such molecules. Polar molecules tend to:
- have higher melting points than nonpolar molecules
- have higher boiling points than nonpolar molecules
- be more soluble in water (dissolve better) than nonpolar molecules
- have lower vapor pressures than nonpolar molecules
We will examine these ideas more in the next section.
Example 6.13.2
Label each of the following as polar or nonpolar.
a. Water, H2O:
b. Methanol, CH3OH:
c. Hydrogen Cyanide, HCN:
d. Oxygen, O2:
e. Propane, C3H8:
Solution
a. Water is polar. Any molecule with lone pairs of electrons around the central atom is polar.
b. Methanol is polar. This is not a symmetric molecule. The −OH side is different from the other 3 −H sides.
c. Hydrogen cyanide is polar. The molecule is not symmetric. The nitrogen and hydrogen have different electronegativities, creating an uneven pull on the electrons.
d. Oxygen is nonpolar. The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount.
e. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons.
Exercise 6.13.2
Label each of the following as polar or nonpolar.
a. SO3
b. NH3
Answer
a. Nonpolar
b. Polar
Key Takeaways
- The ability of an atom in a molecule to attract shared electrons is called its electronegativity.
- Chemical bonds between atoms with relatively large differences in electronegativity are either ionic or polar covalent, while bonds between atoms with small or no electronegativity differences are nonpolar covalent.
- Molecules can be classified as polar or nonpolar, depending on whether they have an overall molecular dipole or not.
- The polarity of a molecule affects many of its physical properties, such as melting point, solubility in water, and vapor pressure.
Supplemental Video Support
Contributions & Attributions
- StackExchange (thomij)
- Marisa Alviar-Agnew (Sacramento City College)
- Henry Agnew (UC Davis)

