7.1: Solutions – Homogeneous Mixtures
Learning Objectives
- Learn terminology involving solutions.
- Explain the significance of the statement “like dissolves like.”
- Explain why certain substances dissolve in other substances.
Solutions, Solvents, and Solutes
A solution is a homogeneous mixture that is formed when a solute dissolves in a solvent. The major component of a solution is the solvent and the minor component is the solute. By “major” and “minor”, we mean whichever component has the greater or lesser presence by mass or by moles. Sometimes this is confusing, especially with substances with very different molar masses. However, here we will confine the discussion to solutions for which the major component and the minor component are obvious.
Solutions exist for every possible phase of the solute and the solvent. Salt water, for example, is a solution of solid sodium chloride (NaCl) in liquid water (Figure 7.1.1), while air is a solution of gaseous solutes (O2 and other molecules) in a gaseous solvent (N2). In all cases, however, the overall phase of the solution is the same phase as the solvent. Table 7.1.1 lists some common types of solutions, with examples of each.
Table 7.1.1: Types of Solutions
| Solvent Phase | Solute Phase | Example |
| Gas | Gas | Air |
| Liquid | Gas | Carbonated Beverages |
| Liquid | Liquid | ethanol (C2H5OH) in H2O (alcoholic beverages) |
| Liquid | Solid | Salt water |
| Solid | Gas | H2 gas absorbed by Pd metal |
| Solid | Liquid | Hg(ℓ) in dental fillings |
| Solid | Solid | Steel fillings |

Example 7.1.1
A solution is made by dissolving 1.00 g of sucrose (C12H22O11) in 100.0 g of liquid water. Identify the solvent and solute in the resulting solution.
Solution
Either by mass or by moles, the obvious minor component is sucrose, so it is the solute. Water—the majority component—is the solvent.
Exercise 7.1.1
A solution is made by dissolving 3.33 g of HCl(g) in 40.0 g of liquid methyl alcohol (CH3OH). Identify the solvent and solute in the resulting solution.
Answer
solute: HCl(g); solvent: CH3OH
Dissolution and Solvation
Dissolution means the process of dissolving or forming a solution. When dissolution happens, the solute separates into ions or molecules, and each ion or molecule is surrounded by molecules of solvent. The interactions between the solute particles and the solvent molecules is called solvation. A solvated ion or molecule is surrounded by solvent molecules (Figure 7.1.2).
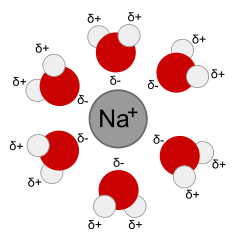
A common solvent is water. When you scuba dive in the ocean, you rinse your gear with water afterwards to remove the salt. The salt dissolves in the water, gets washed away, and then the water evaporates, leaving the gear clean.
“Like Dissolves Like”
Solvents, like all molecules, are either polar or non-polar. A polar solvent has partial negative and positive charges. For instance, water has a partial negative charge on O and a partial positive charge on H. The symbol δ means a partial charge, less than the charge on one proton or electron, such as δ+ or δ–. This helps the solvent interact with (solvate) ions and polar molecules through electrostatic interactions. A non-polar solvent is one that is electrically neutral all over, or almost so. Motor oil and the gasoline in your car are examples of non-polar liquids that could be used as solvents. Non-polar solvents are only good for dissolving non-polar solutes, which is why water, salt and sugar don’t mix into oil.
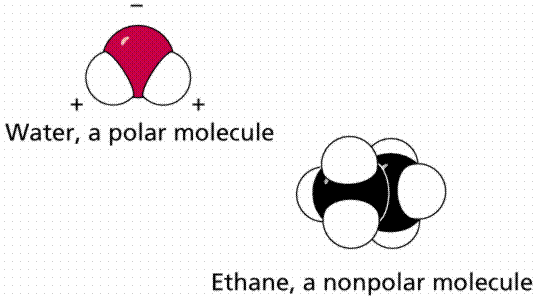
A simple way to predict which compounds will dissolve in other compounds is the phrase “like dissolves like”. What this means is that polar compounds dissolve polar compounds, nonpolar compounds dissolve nonpolar compounds, but polar and nonpolar do not dissolve in each other.
Even some nonpolar substances dissolve in water but only to a limited degree. Have you ever wondered why fish are able to breathe? Oxygen gas, a nonpolar molecule, does dissolve in water—it is this oxygen that the fish take in through their gills. The reason we can enjoy carbonated sodas is also due to a nonpolar compound that dissolves in water. These sodas have carbon dioxide gas, CO2, a nonpolar compound, dissolved in a sugar-water solution. In this case, to keep as much gas in solution as possible, the sodas are kept under pressure.
This general trend of “like dissolves like” is summarized in the following table:
Table 7.1.2: Summary of “Like Dissolves Like” Solubility
| Solute (Polarity of Compound) | Solvent (Polarity of Compound) | Dominant Intermolecular Force | Is Solution Formed? |
| Polar | Polar | Dipole-Dipole Force and/or Hydrogen Bond | Yes |
| Non-polar | Non-polar | Dispersion Force | Yes |
| Polar | Non-polar | \ | No |
| Non-polar | Polar | \ | No |
| Ionic | Polar | Ion-Dipole | Yes |
| Ionic | Non-polar | \ | No |
Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. When particles with no charges (nonpolar compounds) are mixed, they will form a solution. However, if substances with charges are mixed with other substances without charges, a solution often does not form. When an ionic compound is considered “insoluble”, it doesn’t necessarily mean the compound is completely untouched by water. All ionic compounds dissolve to some extent. An insoluble compound just doesn’t dissolve in any noticeable or appreciable amount.
What makes a solute soluble in some solvents but not others?
The answer is intermolecular forces. These intermolecular interactions include London dispersion forces, dipole-dipole interactions, and hydrogen bonding. From experimental studies, it has been determined that if molecules of a solute experience the same intermolecular forces that the solvent does, the solute will likely dissolve in that solvent. Thus, sodium chloride—a very polar substance because it is composed of ions—dissolves in water, which is very polar, but not in oil, which is generally nonpolar. Nonpolar wax dissolves in nonpolar hexane, but not in polar water.

Example 7.1.2
Would I2 be more soluble in CCl4 or H2O? Explain your answer.
Solution
I2 is nonpolar. Of the two solvents, CCl4 is nonpolar and H2O is polar, so I2 would be expected to be more soluble in CCl4.
Exercise 7.1.2
Would C3H7OH be more soluble in CCl4 or H2O? Explain your answer.
Answer
H2O, because both experience hydrogen bonding.
Example 7.1.3
Water is considered a polar solvent. Which substances should dissolve in water?
a. methanol (CH3OH)
b. sodium sulfate (Na2SO4)
c. octane (C8H18)
Solution
Because water is polar, substances that are polar or ionic will dissolve in it.
a. Because of the OH group in methanol, we expect its molecules to be polar. Thus, we expect it to be soluble in water.
b. Sodium sulfate is an ionic compound. Recalling the solubility rules, ionic compounds involving sodium cations and most compounds involving sulfate ions are soluble, so we expect sodium sulfate to be soluble in water.
c. Like other hydrocarbons, octane is nonpolar, so we expect that it would not be soluble in water.
Exercise 7.1.3
Toluene (C6H5CH3) is widely used in industry as a nonpolar solvent. Which substances should dissolve in toluene?
a. water (H2O)
b. sodium sulfate (Na2SO4)
c. octane (C8H18)
Answer
Octane (C8H18) will dissolve. It is also non-polar.
Key Takeaways
- Solutions are composed of a solvent (major component) and a solute (minor component).
- “Like dissolves like” is a useful rule for deciding if a solute will be soluble in a solvent.
Contributions & Attributions
- Marisa Alviar-Agnew (Sacramento City College)
- Henry Agnew (UC Davis)
- Emily V Eames (City College of San Francisco)

