5.7: Yields
Learning Objective
- Define and determine theoretical yields, actual yields, and percent yields.
In all the previous calculations we have performed involving balanced chemical equations, we made two assumptions:
- The reaction goes exactly as written.
- The reaction proceeds completely; that is, the reactants are completely converted to products.
In reality, such things as side reactions occur that make some chemical reactions rather messy. For example, in the actual combustion of some carbon-containing compounds, such as methane, some CO is produced as well as CO2. However, we will continue to ignore side reactions, unless otherwise noted. The second assumption, that the reaction proceeds completely, is more troublesome. Many chemical reactions do not proceed to completion as written, for a variety of reasons (some of which we will consider when we talk about acids and bases later in the course). When we calculate an amount of product assuming that all the reactant reacts, we are calculating the theoretical yield, an amount that is theoretically produced as calculated using the balanced chemical reaction.
In many cases, however, this is not what really happens. In many cases, less—sometimes, much less—of a product is made during the course of a chemical reaction. The amount that is actually produced in a reaction is called the actual yield. By definition, the actual yield is less than or equal to the theoretical yield. If it is not, then an error has been made.
Both theoretical yields and actual yields are expressed in units of moles or grams. It is also common to see something called a percent yield. The percent yield is a comparison between the actual yield and the theoretical yield and is defined as:
[latex]percent~yield[/latex] = [latex]\frac{actual~yield}{theoretical~yield}[/latex] x [latex]100\%[/latex]
It does not matter whether the actual and theoretical yields are expressed in moles or grams, as long as they are expressed in the same units. However, the percent yield always has units of percent. Proper percent yields are between 0% and 100%. Again, if percent yield is greater than 100%, an error has been made.
Example 5.7.1
A worker reacts 30.5 g of Zn with nitric acid and evaporates the remaining water to obtain 65.2 g of Zn(NO3)2. What are the theoretical yield, the actual yield, and the percent yield?
Zn(s) + 2 HNO3(aq) → Zn(NO3)2(aq) + H2(g)
Solution
A mass-mass calculation can be performed to determine the theoretical yield. We need the molar masses of Zn (65.39 g/mol) and Zn(NO3)2 (189.41 g/mol). In three steps, the mass-mass calculation is:
[latex]30.5\cancel{g\, Zn}\times \frac{1\, \cancel{mol\, Zn}}{65.39\cancel{g\, Zn}}\times \frac{1\, \cancel{mol\, Zn(NO_{3})_{2}}}{1\cancel{mol\, Zn}}\times \frac{189.41\, g\, Zn(NO_{3})_{2}}{1\cancel{mol\,Zn(NO_{3})_{2}}}=88.3\, g\, Zn(NO_{3})_{2}\nonumber[/latex]
Thus, the theoretical yield is 88.3 g of Zn(NO3)2. The actual yield is the amount that was actually made, which was 65.2 g of Zn(NO3)2. To calculate the percent yield, we take the actual yield, divide it by the theoretical yield, and multiply by 100:
[latex]\frac{65.2\, g\, Zn(NO_{3})_{2}}{88.3\, g\,Zn(NO_{3})_{2}}\times 100\%=73.8\%\nonumber[/latex]
The worker achieved almost three-fourths of the possible yield.
Exercise 5.7.1
Aluminum metal and elemental bromine (Br2) react together to form aluminum bromide. Suppose you start with 25.0 g of aluminum and 100.0 g of bromine and obtain 64.2 g of aluminum bromide. What is the limiting reagent? What is the theoretical yield of aluminum bromide? What is the percentage yield of this reaction?
Answer
Br2 is limiting. The theoretical yield of AlBr3 is 111 g. The percentage yield is 57.8%. (Be sure you are working with a properly balanced equation!)
Chemistry
Many drugs are the product of several steps of chemical synthesis. Each step typically occurs with less than 100% yield, so the overall percent yield might be very small. The general rule is that the overall percent yield is the product of the percent yields of the individual synthesis steps. For a drug synthesis that has many steps, the overall percent yield can be very tiny, which is one factor in the huge cost of some drugs. For example, if a 10-step synthesis has a percent yield of 90% for each step, the overall yield for the entire synthesis is only 35%. Many scientists work every day trying to improve percent yields of the steps in the synthesis to decrease costs, improve profits, and minimize waste.
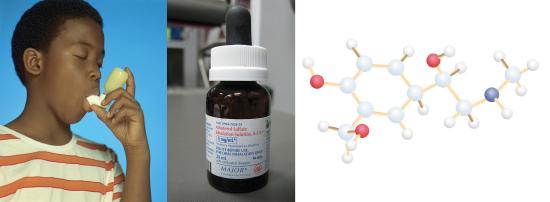
Even purifications of complex molecules into drug-quality purity are subject to percent yields. Consider the purification of impure albuterol. Albuterol (C13H21NO2) is an inhaled drug used to treat asthma, bronchitis, and other obstructive pulmonary diseases. It is synthesized from norepinephrine, a naturally occurring hormone and neurotransmitter. Its initial synthesis makes very impure albuterol that is purified in five chemical steps. The details of the steps do not concern us; only the percent yields do:
| impure albuterol → intermediate A |
percent yield = 70%
|
| intermediate A → intermediate B |
percent yield = 100%
|
| intermediate B → intermediate C |
percent yield = 40%
|
| intermediate C → intermediate D |
percent yield = 72%
|
| intermediate D → purified albuterol |
percent yield = 35%
|
| overall percent yield = 70% × 100% × 40% × 72% × 35% = 7.5% | |
That is, only about one-fourteenth of the original material was turned into the purified drug. This demonstrates one reason why some drugs are so expensive—a lot of material is lost in making a high-purity pharmaceutical.
Key Takeaways
- The theoretical yield is the calculated yield using the balanced chemical reaction. To determine the theoretical yield, you must find the limiting reagent.
- The actual yield is the amount of product that is actually obtained in a chemical reaction when you perform the reaction in lab.
- The percentage yield is a comparison of the actual yield with the theoretical yield.

