Chapter 7 – Practice Problems (Definitions)
- Define solute and solvent.
-
Define saturated, unsaturated, and supersaturated.
-
A solution is prepared by combining 2.09 g of CO2 and 35.5 g of H2O. Identify the solute and solvent.
-
A solution is prepared by combining 10.3 g of Hg(ℓ) and 45.0 g of Ag(s). Identify the solute and solvent.
- Use the associated table to determine the following: decide if a solution containing 45.0 g of NaCl per 100 g of H2O is unsaturated, saturated, or supersaturated.
Solute Solubility (g per 100 g of H2O at 25°C) AgCl 0.00019 CaCO3 0.0006 KBr 70.7 NaCl 36.1 NaNO3 94.6 - Use the table in question 5 to determine the following: decide if a solution containing 0.000092 g of AgCl per 100 g of H2O is unsaturated , saturated, or supersaturated.
- Would the solution in Exercise 5 be described as dilute or concentrated? Explain your answer.
- Would the solution in Exercise 6 be described as dilute or concentrated? Explain your answer.
- Identify a solute from the table in question 5 – Solubilities of Some Ionic Compounds, whose saturated solution can be described as dilute.
- Identify a solute from the table in question 5 – Solubilities of Some Ionic Compounds, whose saturated solution can be described as concentrated.
- Which solvent is Br2 more likely soluble in—CH3OH or C6H6?
- Which solvent is NaOH more likely soluble in—CH3OH or C6H6?
- Compounds with the formula CnH2n + 1OH are soluble in H2O when n is small but not when n is large. Suggest an explanation for this phenomenon.
- Glucose has the following structure:
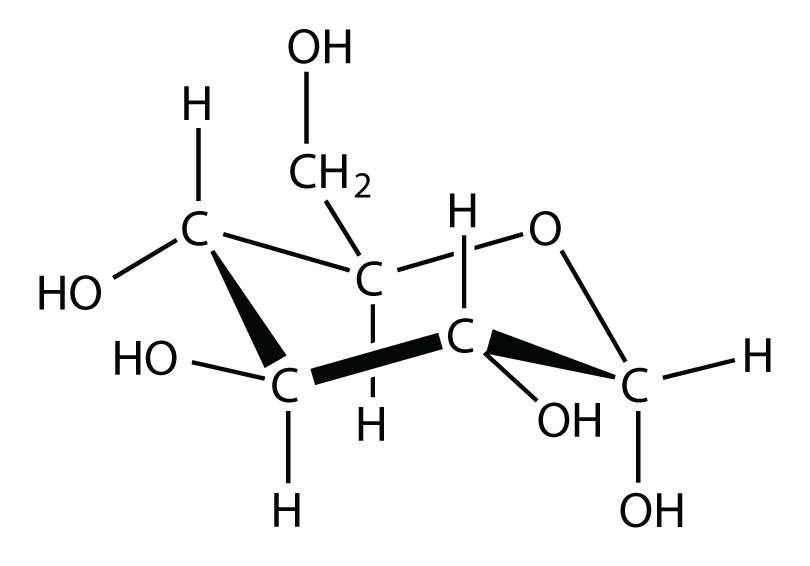
What parts of the molecule indicate that this substance is soluble in water?

