Elimination Reactions
Nucleophilic substitution reaction is not the only possible reaction for alkyl halides and other substrates with a good leaving group. These substrates could also undergo elimination reaction. In an elimination reaction, a small molecule (XY) is removed from two adjacent atoms of the reactant, and a multiple bond is formed in the product:
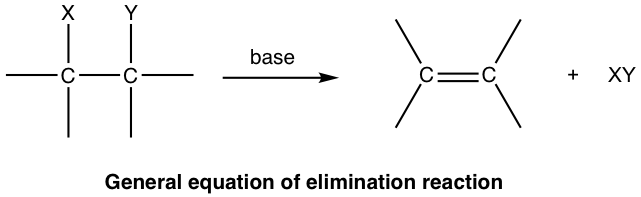
For elimination reaction of alkyl halides, the major product alkene is produced together with the small HX (X is halogen) molecule, which is the side inorganic product. Such reaction with removal of a proton and a halide ion is called dehydrohalogenation. Dehydrohalogenation is a commonly applied method for the synthesis of alkene.
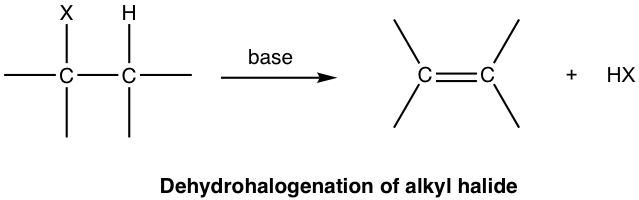
In the discussions of elimination reaction, the carbon atom that bonded to the leaving group (halogen for alkyl halide) is called the alpha (α) carbon atom, and the carbon atom adjacent to α-carbon is called the beta (β) carbon atom. In dehydrohalogenation, it is the hydrogen atom on β-carbon that is eliminated together with halogen, as HX, therefore the reaction is often called as β-elimination, or 1,2-elimination. (The number 1,2- indicate that the atoms being removed are on two adjacent carbons, not necessarily means C1 and C2 atoms.)
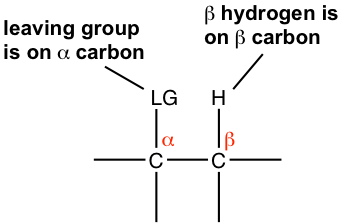
Beside the substrate, a base is required for elimination reactions, other than the nucleophile for substitution. Similar to substitution reaction, elimination reactions also have different rate laws, and therefore involve different mechanisms. The elimination mechanisms are E1 and E2. You may expect that E1 is the unimolecular reaction with first order rate law, and E2 is the second order bimolecular reaction. That is correct. We will go through the mechanism in details first, then compare between elimination and substitution.
Module Level Learning Objectives
Type your learning objectives here.
- First
- Second

