6 Summary of Isomers
Chapter 6 Learning Objectives
- Distinguish constitutional and stereoisomers.
- Be able to identify a pair of compounds as identical, different or isomeric.
6.1 Introduction
There are two major types of isomers, constitutional isomers and stereoisomers. We are familiar with constitutional isomers, and will focus on stereoisomers in this chapter. Stereoisomers are molecules with same bonding, but groups are in different spatial arrangement. At beginning of this chapter, we will learn more about geometric isomers in alkenes and the E/Z naming system. Then we will move on to a brand new category of stereoisomers, the isomers with chirality center. The flowchart here show the correlation and difference between different types of isomers (pay attention to the definitions included) and provides useful guideline for the learning.
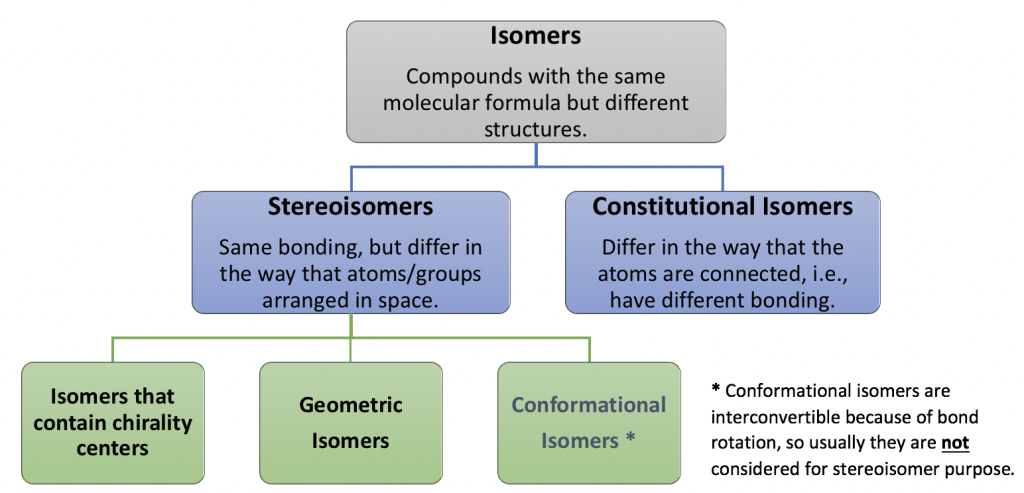
Figure 6.1: Types of Isomers
Additional Resources:
- A brief guide to types of isomerism in organic chemistry.
- View this video on structural isomers.
Key Takeaways: Structural Isomers
Constitutional/structural isomers have
- Different IUPAC names
- Same or different functional groups
- Different physical properties
- Different chemical properties
Key Takeaways: Stereoisomers
Stereoisomers
- Differ only in the way the atoms are oriented in space.
- Have identical IUPAC names (except for a prefix like cis or trans or R or S).
- Always have the same functional group(s).
Exercises 6.1: Types of Isomers
Identify whether each of the following are constitutional isomers, stereoisomers, the same compound or different compounds.
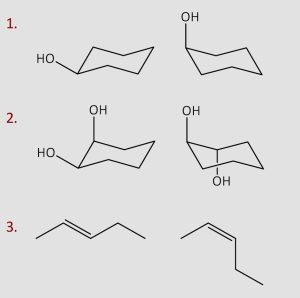
Answers:
1 – same
2 – stereoisomers
3-stereoisomers
a group of atom(s) responsible for physical and chemical properties of an organic molecule

