9 Fisher Projection
Chapter 9 Learning Objectives
- Be able to draw Fisher projection formulas.
- Be able to assign R or S configuration to Fisher formulas.
9.1 Introduction
For the discussions so far, the perspective formula with solid and dashed wedges have been used to represent the 3D arrangement of groups bonded to a chirality center. Other than that, there is another broadly applied formula for that purpose, that is the Fisher projection. A Fisher projection is a shortcut for showing the spatial group arrangement of a chirality center, it is more easily to be drawn and recognized, and is particularly useful for showing the structures with more than one chirality centers.
In Fisher projection, the chirality center is shown as the intersection of two perpendicular lines. The horizontal lines represent the bonds point out of the plane, and the vertical lines represent the bonds that point behind the plane.
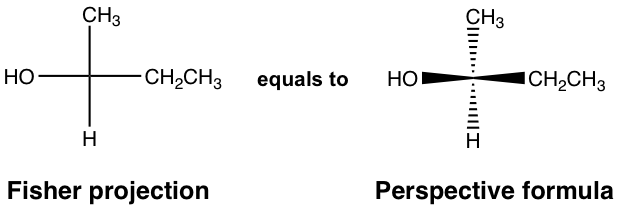
9.2 Assigning R/S Configuration in Fisher projection
Taking the following compound as an example:
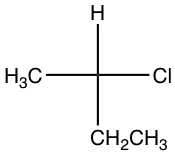
- Assign group priority as we usually do.
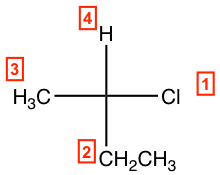
- If the lowest priority group (#4 group) is on a vertical bond, determine the priority decrease direction from #1→#2→#3 as usual to get the configuration, clockwise is R and counterclockwise is S.
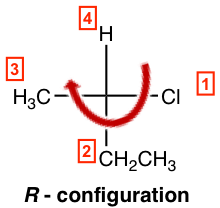
So, the example here is a R-isomer, and the complete name of the compound is (R)-2-chlorobutane.
- If the lowest priority group is on a horizontal bond (as the case in the following structure), determine the priority decrease direction as in step 2, then reverse the answer to opposite way, to get the final configuration.
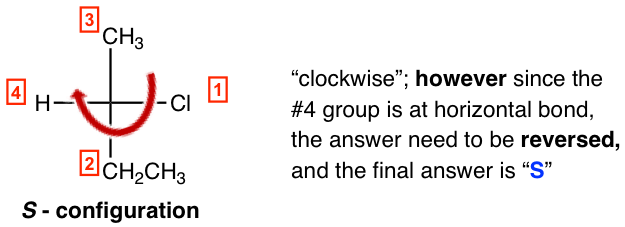
So, the example here is a S-isomer, and the complete name of the compound is (S)-2-chlorobutane.
You may use the mnemonic, Horizontal = Horribly wrong; Vertical = very correct
Exercise 9.1
Explain that why in step 3 of the above procedure, the answer should be reversed to get the final (actual) configuration?
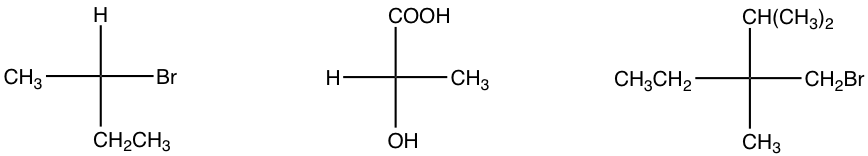
Exercises 9.2 Indicate the configuration of the following structures.
9.3 Properties of Fisher projection
1. One switch (interchange) of two groups in a Fisher projection invert the configuration, two switches bring the original isomer back.
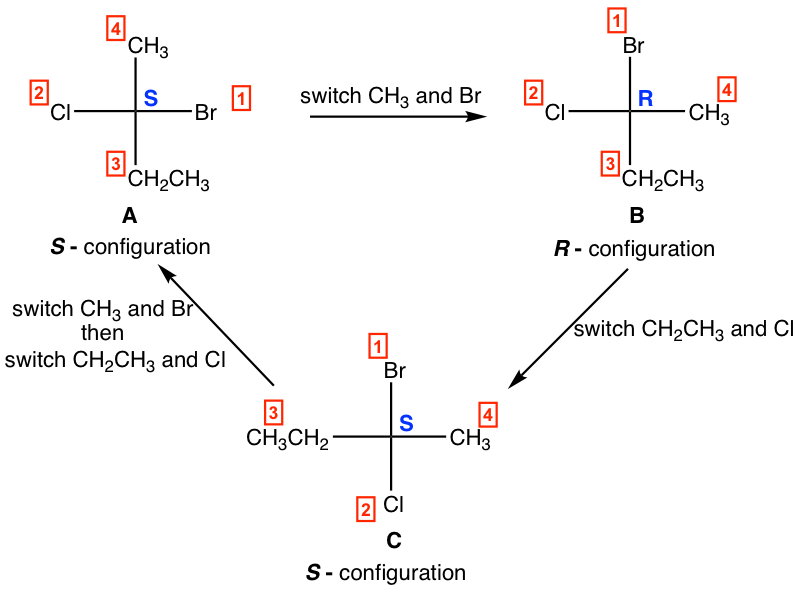
For above structures:
- one switch of A leads to B, A and B are enantiomers;
- one switch of B leads to C, B and C are enantiomers;
- two switches of C leads to A, A and C are identical.
2. Rotate the Fisher projection 180º get same structure, with the configuration retained.
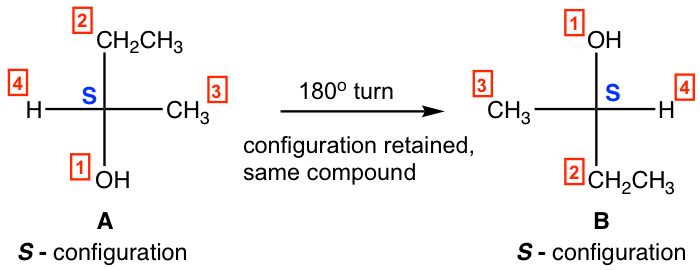
- 180º rotation of A leads to B, A and B are identical.
3. Rotate the Fisher projection 90º get the configuration inverted.

- 90º rotation of A leads to B, A and B are enantiomers.
Do NOT rotate the Fisher projection 90º, unless you have to. Keep in mind that the configuration get inverted by 90º rotation.

