37 Structure Determination Practice
Chapter 37 Learning Objectives
Be able to solve for the structure of a given molecule based on spectral data provided.
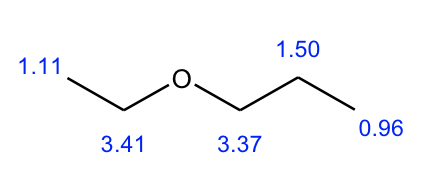
1H NMR provides a powerful tool for determining the structure of unknown compound. Other than that 1H NMR, additional information includes molecular formula, IR and 13C NMR spectrum are usually provided as well. Solving the structure of an unknown compound based on all the given information is an important type of question we will work on for this chapter. We will take the C5H12O constitutional isomer example to go through the strategy for solving this type of question.
Approach:
Step 1: Calculate the degree of unsaturation based on the given molecular formula, and get hints about structure/functional group according to the degree of unsaturation (n = number of C atoms; X = number of H atoms). This is usually the first step to solving this type of question.
Degree of unsaturation =![]()
From what we learned about the degree of unsaturation, zero degree means there is no any ring nor double bond in the structure, that means all the compounds in this question have open chain structures with single bonds only. With one oxygen atom involved, the possible functional group therefore will be open chain alcohol, or open chain ether.
Step 2: Narrow down the possible functional groups with IR information.
IR indicate that there is noany strong bands at above 3000 cm-1 for the compound, that exclude the option of alcohol, so the only choice left is the open chain ether.
Step 3: Use available spectroscopy data (mainly 1H NMR, with 13C NMR as supporting if available) to identify discrete parts of the structure.
Step 4: Try to put the pieces of the puzzle together, and double check if everything fit the available data.
Step 3 and 4 are the most challenging parts since there is no simple rule to follow about how to do that. It takes practices to do the interpretation of 1H NMR signals and translating that into the structure of unknown compound. Checking the four aspects of 1H NMR – number of peaks, chemical shift, peak integration and multiplicity pattern). The relative integration areas are given for this question to make it bit easier.
Solutions:
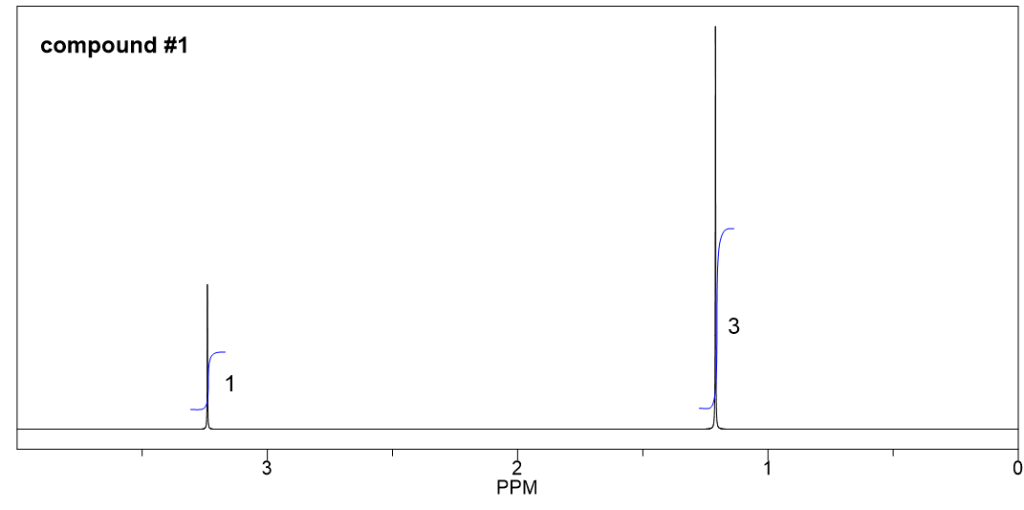
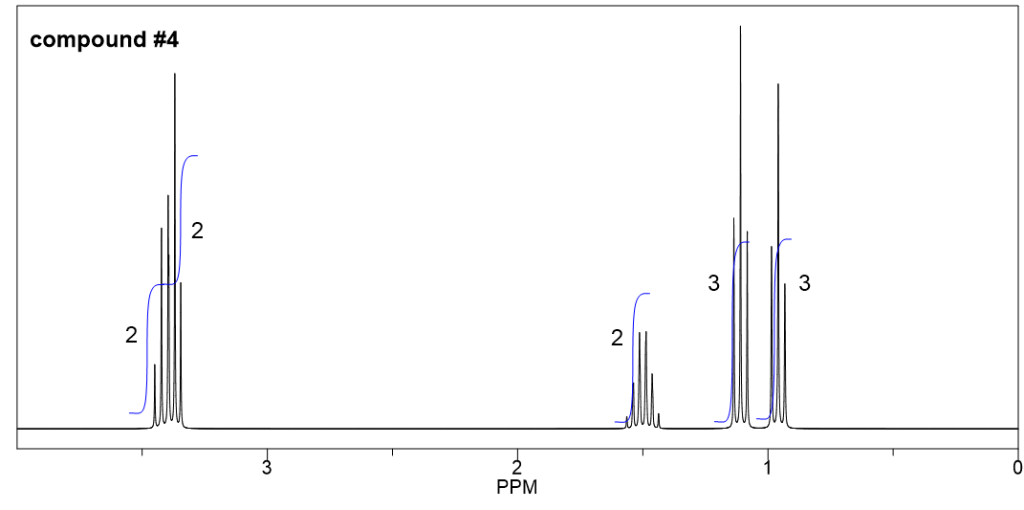
Compound 1:
We can start with the simplest spectrum that have least signals:
- There are only two signals (both are singlet) in this spectrum, indicate that there are two sets of non-equivalent hydrogens.
- The integrations of the two signals are 3 and 1, means the ratio of the number of hydrogens in these two sets are 3:1. And since there are total 12 hydrogens, the actual number of hydrogens should be 9 and 3 in each group.
- 3 hydrogens imply a CH3 methyl group, and 9 hydrogens could be three CH3 groups. Also since all the 9 hydrogens are equivalent, that means the three CH3 groups are equivalent. The only way to have three equivalent CH3 groups is that there is a t-butyl group.
- So the structure is the ether with a methyl group and a t-butyl group connected with the oxygen atom.
- The structure of compound 1 is given below, with the chemical shift valued included.
For the remaining compounds, the integration for each signal could be a very good starting point, since generally the integration value indicates the possible structural unit like CH3, CH2 or CH. Then the structural units can be put together in a logical way like putting pieces of a puzzle together.
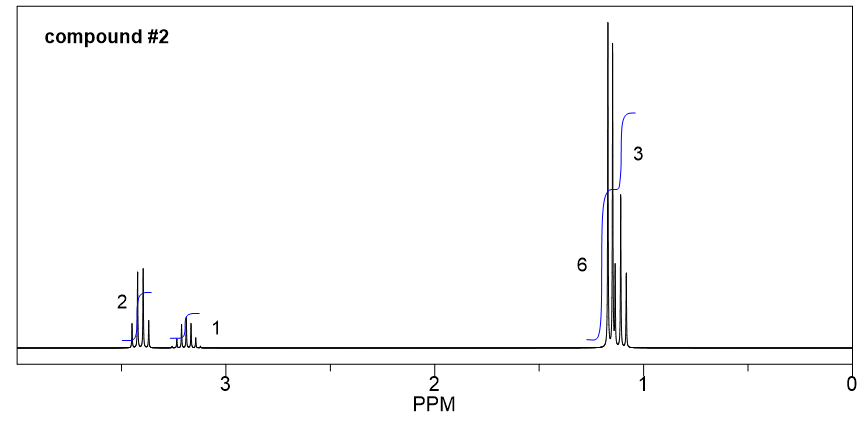
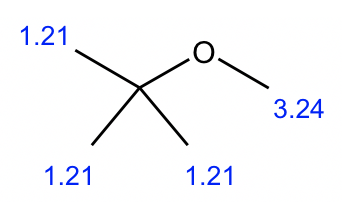
Compound 2:
- Based on the integration, it is determined that there are:
- one CH3 group show a triplet;
- two equivalent CH3 groups show a doublet;
- one CH group show a multiplet;
- one CH2 group show a quartet.
- The triplet CH3 could connect with quartet CH2 as a CH2CH3 ethyl group, that makes sense based on the splitting pattern.
- Also, the two equivalent CH3 groups with a CH could give an isopropyl group, that is consistent to the splitting pattern.
- So the overall structure of compound 2 is isopropyl ethyl ether.
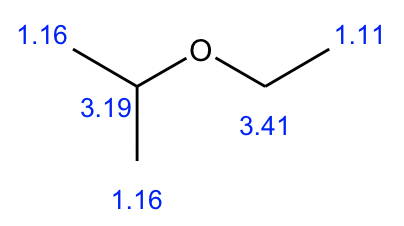
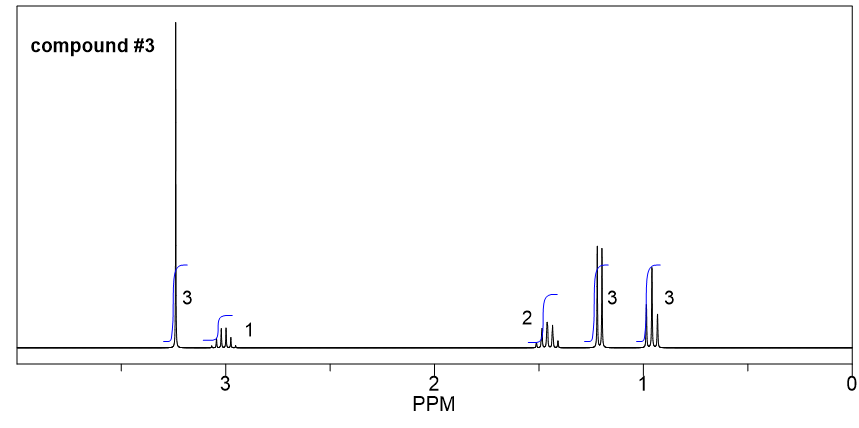
Compound 3:
- Based on the integration, it is determined that there are:
- one CH3 group show a triplet;
- one CH3 groups show a doublet;
- one CH2 group show a multiplet;
- one CH group show a quartet;
- one CH3 group show a singlet.
- The singlet means the CH3 has no any other hydrogens bonded on adjacent atoms, so the CH3 group should be bonded with the oxygen atom, and the value of chemical shift (about 3.2 ppm) confirms.
- The triplet CH3 could connect with quartet CH2 as a CH2CH3 ethyl group, that makes sense based on the splitting pattern.
- The doublet CH3 groups should connect with a CH group, that is consistent to the splitting pattern.
- The chemical shift (about 3 ppm) and splitting of the CH group (quartet) indicate it should connect to the oxygen atom.
- Put all the above pieces together, the structure of compound 3 is sec-butyl methyl ether.
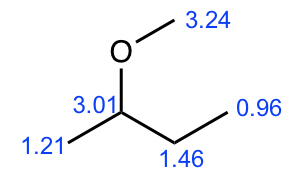
Compound 4
- Based on the integration, it is determined that there are:
- one CH3 group show a triplet;
- another CH3 groups show a triplet;
- one CH2 group show a multiplet;
- two CH2 groups with signals overlapping
- The two CH3 groups both as triplet indicate that they both connect with CH2, so there are two ethyl CH2CH3 groups in the structure, and they are not equivalent.
- Therefore there is only one more CH2 group left.
- There is only one possible structure with two CH2CH3 groups, one CH2 group and one oxygen atom, so the structure of compound 4 is ethyl methyl ether.