25 Stereochemistry for Halogenation of Alkanes
Chapter 25 Learning Objectives
Predict the stereochemistry of the product(s) of radical halogenation of alkanes.
In the structure of carbon radical, carbon has three bonds and one single electron. Based on VSEPR, there are total four electron groups, radical should be in tetrahedral shape. However, experiment evidence indicate that the geometric shape of most alkyl radical is trigonal planar shape, with the carbon in sp2 hybridization, and there is one single unpaired electron in the unhybridized 2p orbital.
We will take the bromination reaction of (±)-3-methylhexane to explain the stereochemistry. The experiment results indicate that the racemic mixture of R and S 3-bromo-3-methylhexane were obtained with the bromination.
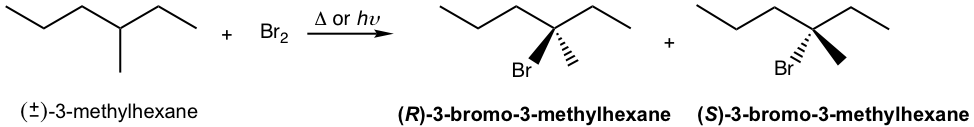
This can be explained by the stereochemistry of the propagation steps in the mechanism. The carbon radical generated in step 1 is in trigonal planar shape as mentioned earlier. When the radical reacts with bromine in step 2, the reaction can occur at either side of the plane. Because both sides are identical, the probability of the reaction by either side is the same, therefore equal amount of the R– and S– enantiomer are obtained as a racemic mixture.
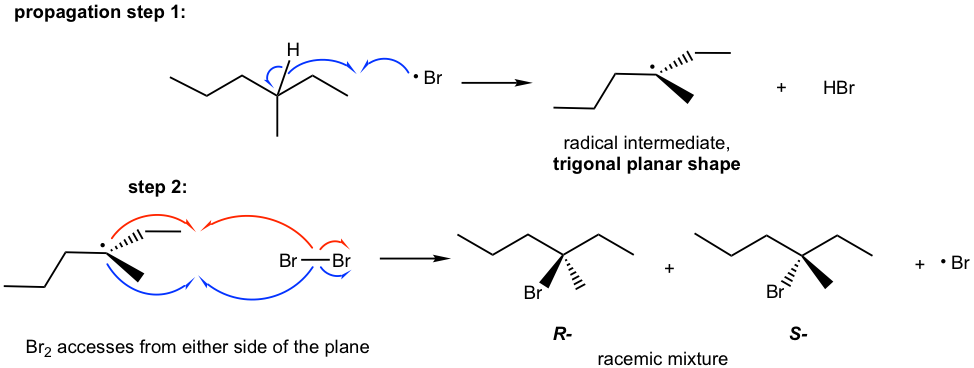
The stereochemistry of the radical substitution is similar to that of SN1 reaction, because both carbon radical and carbocation are in trigonal planar shape.
Examples
Show the bromination product(s) with stereoisomers when applied.
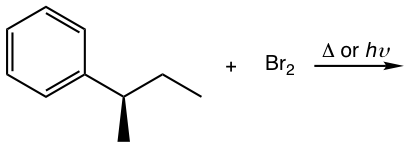
Solutions:
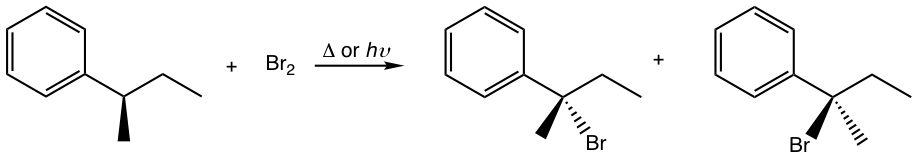
The racemic mixture is obtained.
Exercises 25.1: Show all the mono-chlorination products of butane with any stereoisomers when applied.
![]()

