1 Structure and Bonding
Chapter 1 Learning Objectives
- Define organic chemistry
- What makes carbon special?
- Be able to distinguish ionic and covalent bonding.
- Be able to distinguish nonpolar and polar covalent bonds.
- Be able to draw Lewis structures of organic molecules.
- Be able to determine the shape of a given organic molecule.
- Be able to calculate the formal charge on an atom.
- Be able to explain the hybridization theory and bonding of carbon in organic compounds.
- Be able to draw expanded, condensed and skeletal structures of organic compounds.
- Be able to draw resonance structures.
1.1. Introduction
Organic chemistry is an essential and fascinating branch of science that explores the structure, properties, and reactions of carbon-based compounds, the very building blocks of life. Many students begin this course as a prerequisite for health-related programs, while others arrive with a natural curiosity and eagerness to explore the subject. Whether you’re continuing your education, returning after a long break, or simply seeking to understand the world on a molecular level, organic chemistry offers a unique lens through which to view the world around you.
You may have heard both challenging and inspiring accounts about this course. Indeed, organic chemistry has a reputation for its rigor—but it is also immensely rewarding. At its core, organic chemistry is the study of life. From the DNA in our cells to the medications that treat disease, from the foods we eat to the fuels that power our lives, organic chemistry plays a pivotal role in advancing human health, industry, and technology. Understanding organic chemistry equips you not only with knowledge, but also with critical thinking and problem-solving skills that are essential in a wide range of scientific and medical fields.
As you embark on this journey, prepare to be challenged, inspired, and amazed by the molecular intricacies that sustain and shape our world.
This article highlights some everyday applications of organic chemistry. Listen to the TedTalk that tells you why it is a smart move to take this course.
1.2 How did it all begin?
For a longest time, scientists assumed that organic compounds cannot be made from non-living matter. This became popularly known as the ‘vital theory’, an unseen vital force that helped make organic compounds from living things. Friedrich Wohler, a 28 year old chemist, synthesized the first organic compound in 1828 by heating ammonium cyanate, an inorganic compound, to produce urea, an organic compound (Scheme 1.1). This brought an end to the vital theory.
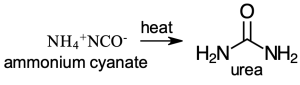
Scheme 1.1: Pyrolysis of Ammonium Cyanate
What is the traditional definition of organic chemistry?
Organic chemistry is the study of the synthesis, structure, reactivity and properties of a large group of chemical compounds primarily consisting of carbon. Watch this CrashCourse video to obtain additional insight into the definition of organic chemistry.
What is so special about carbon?
The remarkable versatility of carbon lies in its electronic structure and its position in the periodic table (Figure 1.1). As a Group 4A (or Group 14) element, carbon has four valence electrons, allowing it to form four strong covalent bonds with other atoms. This tetravalency is key to carbon’s ability to act as a central building block in countless molecular structures. One of carbon’s most extraordinary properties is its ability to bond with other carbon atoms. These bonds can form stable chains, branched frameworks, and rings of various sizes. Carbon atoms can also engage in multiple bonding, forming double and triple bonds that contribute to structural diversity and chemical reactivity. Because of these unique bonding capabilities, carbon can create an immense variety of compounds, ranging from simple molecules like methane (CH₄), which contains a single carbon atom, to highly complex macromolecules such as DNA, which may contain over 100 million carbon atoms. This unparalleled capacity for forming diverse and stable compounds makes carbon the foundation of organic chemistry and, by extension, life itself.
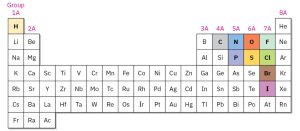
Figure 1.1: Position of Carbon
Here is a quick look at key terms you learned in fundamental chemistry course. These terms will be used often in organic chemistry.
Exercises
-
What is the ground-state electron configuration of each of the following elements:
(a) Oxygen (b) Nitrogen (c) Sulfur
-
How many electrons does each of the following biological trace elements have in its outermost electron shell?
(a) Magnesium (b) Cobalt (c) Selenium
Answers:
The ground-state electron configuration describes the arrangement of electrons around the nucleus of an atom in its lowest energy state.
(a) Oxygen (O, atomic number 8):
Electron configuration: 1s² 2s² 2p⁴
(b) Nitrogen (N, atomic number 7):
Electron configuration: 1s² 2s² 2p³
(c) Sulfur (S, atomic number 16):
Electron configuration: 1s² 2s² 2p⁶ 3s² 3p⁴
To determine the number of valence electrons, we look at the electrons in the outermost (highest principal quantum number) shell.
(a) Magnesium (Mg, atomic number 12):
Electron configuration: 1s² 2s² 2p⁶ 3s²
Valence electrons: 2 (in the 3s subshell)
(b) Cobalt (Co, atomic number 27):
Electron configuration: [Ar] 4s² 3d⁷
Valence electrons: 2 (from the 4s subshell)
(Note: Though 3d electrons participate in bonding, the outermost shell by principal quantum number is the 4s.)
(c) Selenium (Se, atomic number 34):
Electron configuration: [Ar] 4s² 3d¹⁰ 4p⁴
Valence electrons: 6 (2 from 4s and 4 from 4p
1.3 Chemical Bonding: Ionic bonds and covalent bonds
Elements bond with one another to achieve greater stability, often by attaining a full outer electron shell. Two primary types of chemical bonds enable the formation of stable compounds: ionic bonds and covalent bonds.
In an ionic bond, electrons are transferred from one atom to another. This typically occurs between metals on the left side of the periodic table and nonmetals on the right. Metals, such as sodium (Na), lose electrons to become positively charged cations, while nonmetals, like chlorine (Cl), gain electrons to become negatively charged anions. The resulting electrostatic attraction between these oppositely charged ions holds the compound together.
A classic example is sodium chloride (NaCl). In this compound, a sodium atom donates one electron to a chlorine atom. This electron transfer results in the formation of Na⁺ and Cl⁻ ions, which are strongly attracted to each other, forming a stable ionic lattice (Figure 1.2).
In contrast, covalent bonds (discussed in the next section) involve the sharing of electrons between atoms, commonly seen between nonmetals.
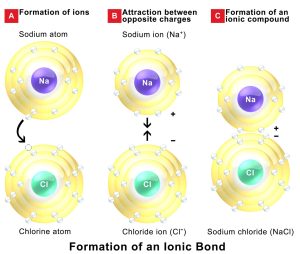
Figure 1.2: Formation of an Ionic Bond
Credits: Wikimedia Commons; This file is licensed under the Creative Commons Attribution-Share Alike 4.0 International license.
On the other hand, covalent bonds result from sharing of electrons. In the case of HF, both hydrogen and fluorine atoms share an electron each to form a bond between them (Figure 1.3). Ionic bonds occur between metals and nonmetals whereas covalent bonds occur between nonmetals. Covalent bonding is the mode of bonding for organic compounds.
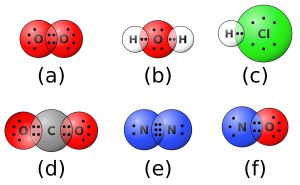
Figure 1.3: Formation of a Covalent Bond
Credits: Wikimedia Commons; This file is licensed under the Creative Commons Attribution-Share Alike 4.0 International license.
Watch this TedEd video that explains why atoms bond? How do they decide if they should bond ionically or covalently?
Key Takeaways: Chemical Bonding
- Bonding is the joining of two atoms in a stable arrangement.
- Through bonding, atoms attain a complete outer shell of valence electrons.
- Through bonding, atoms attain a stable noble gas configuration.
- Ionic bonds result from the transfer of electrons from one element to another.
- Covalent bonds result from the sharing of electrons between two nuclei.
- Organic compounds are formed by covalent bonding.
1.4 Electronegativity
Electronegativity is the property of an atom to attract the shared pair of electrons. Electronegativity values are used as a guideline to indicate whether the electrons in a bond are shared equally or unequally between two atoms. Sharing of electrons is similar to two people playing tug of war, who pulls the hardest.

Linus Pauling introduced this fundamental idea of electronegativity and formulated a scale of 0 to 4, where 4 is the value given to the most electronegative atom (Figure 1.4).
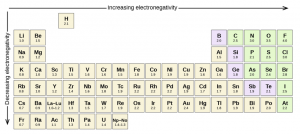
Figure 1.4: Pauling’s Scale of Electronegativity
Credits: Electronegativity and Polarity. (2020, October 12). Retrieved June 28, 2021, from https://chem.libretexts.org/@go/page/210716; ” Electronegativity and Polarity” by LibreTexts is licensed under CC BY .
When electrons are equally shared, the bond is said to be nonpolar covalent. For example, in a chlorine molecule, electrons are shared equally between two chlorine atoms (Figure 1.5).
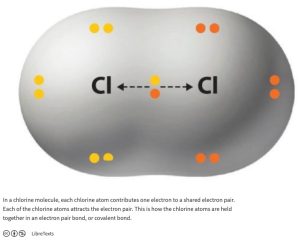
Figure 1.5: Chlorine-Chlorine Non-polar Covalent Bond
On the other hand, in the H—Cl bond, the electrons are pulled away from H (2.1) toward Cl (3.2), the element of higher electronegativity (Figure 1.6). The bond is polar, or polar covalent. The bond is said to have dipole; that is, a separation of charge. When differences in electronegativity result in unequal sharing of electrons, the bond is polar, and is said to have a “dipole”.
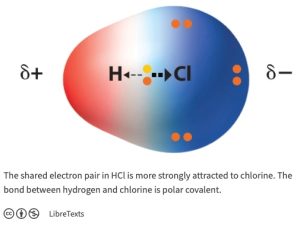
Figure 1.6: Hydrogen-Chlorine Polar Covalent Bond
Credits: Electronegativity and Polarity. (2020, October 12). Retrieved June 28, 2021, from https://chem.libretexts.org/@go/page/210716; ” Electronegativity and Polarity” by LibreTexts is licensed under CC BY .
The direction of polarity in a bond is indicated by an arrow with the head of the arrow pointing towards the more electronegative element. The tail of the arrow, is drawn towards the less electronegative element. Alternatively, a partial positive charge sign, deltaa plus, is placed on the less electronegative element and a partial negative charge, delta minus, is placed on the more electronegative element. It is important to remember that fluorine is the most electronegative element and has a value of 4.0 on the Pauling’s scale of electronegativity. Oxygen is the second most electronegative element. It is important to note that although there is an electronegativity difference between carbon and hydrogen, the difference is too small, and hence the bond is considered non-polar.
To determine the nature of the bond, one may use the following scale (Figure 1.7 and Figure 1.8).
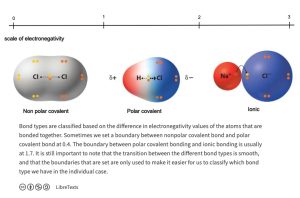
Figure 1.7: Scale to Determine the Nature of a Chemical Bond
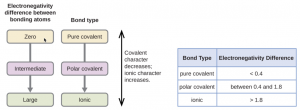
Figure 1.8: Types of Chemical Bond
Additional Resource:
View the following video to understand the relationship between electronegativity and polarity of molecules.
Credits: Crash Course Chemistry; Crash Course Chemistry: Crash Course is a division of Complexly and videos are free to stream for educational purposes.
The O-H bond is a common bond found in many organic molecules. It is a polar covalent bond and the polarity of -OH bonds is responsible for imparting many interesting properties to organic molecules. Learn more about the strange behavior of water due to O-H bonds from this video:
Credits: Ted-Ed; Ted-Ed is an extension of Ted and its mission is creating lessons worth sharing.
Watch these helpful free-access videos from Khan Academy on various aspects of chemical bonding.
Key Takeaways: Polarity and Electronegativity
- Electronegativity is the ability of an atom to attract a shared pair of electrons.
- Based on Pauling’s scale of electronegativity, fluorine is the most electronegative element with a value of 4.0. Oxygen is the second most electronegative element.
- If two atoms that share a bond do not differ in their electronegativities (<0.4), they form a nonpolar covalent bond.
- If two atoms that share a bond differ in their electronegativities (0.5 – 1.8) , they form a polar covalent bond.
- If the elements that form a bond differ in their electronegativities greater than 1.8, they form an ionic bond.
- Covalent bonds are formed between nonmetals while ionic bonds are formed between a metal and a nonmetal or between a cation and an anion.
- Predominant mode of bonding in organic compounds is covalent bonding.
Examples
Look at the following electrostatic potential map of methylamine, a substance responsible for the odor of rotting fish, and tell the direction of polarization of the C–N bond:
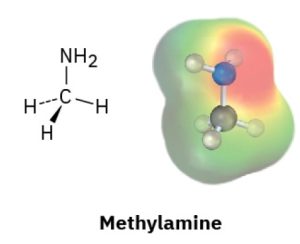
Answer:
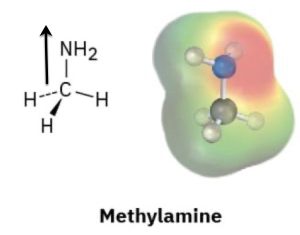
To determine the direction of polarization of the C–N bond in methylamine (CH₃NH₂) using an electrostatic potential map, follow this guideline:
-
Red regions indicate electron-rich areas (higher electron density, partial negative charge δ⁻).
-
Blue regions indicate electron-poor areas (lower electron density, partial positive charge δ⁺).
In the case of methylamine:
-
The nitrogen (N) atom is more electronegative than the carbon (C) atom.
-
On the electrostatic potential map, the region around the nitrogen will appear more red, indicating a partial negative charge (δ⁻).
-
The region near the carbon bonded to nitrogen will appear less red or slightly blue, indicating a partial positive charge (δ⁺).
Answer:
The C–N bond is polarized toward the nitrogen atom, meaning electrons are drawn from carbon toward nitrogen.
So, the direction of polarization is:
C (δ⁺) → N (δ⁻).
Examples
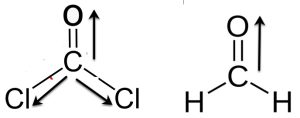
Phosgene, Cl2CO, has a smaller dipole moment than formaldehyde, H2CO, even though it contains electronegative chlorine atoms in place of hydrogen. Explain.
Answer: There are two C-Cl dipoles that cancel the C=O dipole. Therefore, phosgene has a smaller dipole moment. However, in formaldehyde the two C-H bonds are nonpolar and the net resultant dipole moment is from C=O bond.
Examples
Fluoromethane (CH3F, μ = 1.81 D) has a smaller dipole moment than chloromethane (CH3Cl, μ =1.87 D) even though fluorine is more electronegative than chlorine. Explain.
Answer: Dipole moment is a product of charge and distance separating the charges. C-F is much shorter than C-Cl bond (due to better overlap of C-F orbitals). Due to a shorter distance, fluoromethane has a smaller dipole moment.
Examples
Methanethiol, CH3SH, has a substantial dipole moment (μ = 1.52) even though carbon and sulfur have identical electronegativities. Explain.
Answer: C-S bond is long and this increases the dipole moment.
Exercises
- Identify the following bonds as “polar” or “non-polar”: C-C, C-H, B-F, O-O, C=N
- Rank the following bonds in order of increasing bonding polarity: C—S, C—O, C—F (referring to the trend of EN, you do not need to use the exact EN values).
Answers:
- Identify whether the following bond is “polar” or “non-polar”.
C-C: non-polar C-H: non-polar (very close electronegativity for C and H)
B-F: polar O-O: non-polar C=N: polar
2. Rank the following bonds in the order of increasing bonding polarity: C—S, C—O, C—F (referring to the trend of EN, no need to use the exact EN values).
bonding polarity: C—S < C—O < C—F
Key Takeaways
Notes about electronegativity values for Organic Chemistry purposes:
- It is much more important to know the trend of EN than to memorize the values. The trend is that EN values decrease along the group from top to bottom and increase along the period from left to right (the trend mainly works for Main Group elements, not transition metal elements).
- It is very useful (although not mandatory) to know the EN values of a few select elements: F (4.0, highest), O (3.5), N (3.0), C (2.5) and H (2.1).
- The EN of C (2.5) and H (2.1) is close, which makes the C-H bond (the bond involved in all organic compounds) technically non-polar.
1.5 Lewis Structures and Shapes of Molecules (VSEPR theory)
Lewis structures are a convenient way to show the distribution of electrons in a molecule. Drawing Lewis structures correctly is a necessary step to later on write correct organic structures using condensed and line drawings, the common methods by which organic structures are drawn.
The following table shows the 'normal' bonding modes of some important elements, often encountered in organic compounds.
| Element | Carbon | Nitrogen | Oxygen | Halogen |
| Number of Bonds | 4 | 3 | 2 | 1 |
| Lone Pairs | 0 | 1 | 2 | 3 |
Exercise: Draw a valid Lewis structure for the following. Include all lone pair electrons and non-zero formal charges.
A) C2H6 B) C2H4 C) C2H2 D) CH3COOH E) CH3COCH3
Answers: (coming soon)
Terms used in Lewis structures (see example of F2):
- Bonding pair: The pair of valence electrons involved in a covalent bond. The covalent bonds are drawn as short lines in this book, and one covalent bond means one pair of bonding electrons, that is, 2 electrons. Single bonds and multiple bonds (double or triple bonds) may be involved.
- Lone pair: The pairs of valence electrons not involved in a covalent bond. Lone pair electrons can also be called non-bonding electron pairs.
Exercises
Why is the following structure not the best way to show the Lewis structure of CO2 ?
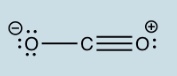
Answer: Because the formal charges are not minimized in the above structure. The formal charge in the best Lewis structure of CO2 are all zero, and the best Lewis structure of CO2 is shown here:
![]()
1.6 VSEPR Model
Why do we need this model? The VSEPR theory helps a chemist to predict the electron geometry, electron geometrymolecular shape, bond angle and the hybridization of the central atom in a molecule. How does this knowledge help us? Watch this video to find out.
For a more detailed lecture on VSEPR theory, visit MIT OpenCourseWare for this recorded lecture.
Three common shapes are encountered in organic compounds. These are listed in Table 1.1.
*A lone pair is considered as one group. A double bond (and a triple bond) is considered as one group. An odd electron is not considered as a group.
| Number of Groups around the Central Atom* | Molecular Shape |
| 4 | tetrahedral |
| 3 | trigonal planar |
| 2 | linear |
Table 1.1: VSEPR Theory
1.7 Calculating Formal Charge
All atoms in a molecule may not be uncharged. Charges affect the course of a chemical reaction, therefore, it is important to calculate the charge and know its location. Formal charge can help us do this. Formal charge is the charge assigned to individual atoms in a Lewis structure. Formal charge is calculated as follows.
The purpose of formal charges is to compare the difference between the number of valence electrons in the free atom and the number of electrons the atom “owns” when it is bonded. The smaller the difference, the “happier” (more stable) the atom is. The atom owns all of the lone pair (non-bonding) electrons and half of the bonding (shared) electrons, which is why the formula is given in the way shown in the formula.
Formal charges can be used as guidelines to determine the plausibility of Lewis structures by comparing the stability of non-equivalent resonance structures, which is particularly important for organic species. The rules about formal charges are:
- The sum of the formal charges must equal to the total charge on the molecule or ion.
- Formal charges should be as small as possible (comparing the absolute value of formal charges for such purposes).
- “-” FC usually appears on the most electronegative atoms (with the stronger ability to pull the shared electrons; this atom is “winning” electrons in the sharing).
- “+” FC usually appears on least electronegative atoms (with the weaker ability to pull the shared electrons; this atom is “losing” electrons in the sharing).
- Structures having formal charges of the same sign on adjacent atoms is unlikely.
Additional Resource:
A simple way of calculating formal charges is explained in this video.
Exercises: Determine the formal charge on the heteroatom in each given structure.
1.8 Wave Mechanical Model of the Atom: Our Current Understanding of the Structure of the Atom
Atoms consist of a dense, positively charged nucleus surrounded by negatively charged electrons. These electrons do not follow fixed paths but instead occupy three-dimensional regions in space known as orbitals. Each orbital represents a region where an electron is most likely to be found—specifically, there is a 95% probability of locating the electron within that space.
What about the other 5%? According to the principles of quantum mechanics, electrons behave as both particles and waves. Their exact position cannot be determined at any given moment; instead, they exist within a probability cloud. If an electron is not found within its orbital, it may be momentarily located outside the defined boundary—still governed by the same probabilistic distribution, but in a region of very low probability.
Orbitals differ in both shape and energy level. For example:
-
s orbitals are spherical in shape.
-
p orbitals have a dumbbell shape and come in three spatial orientations: px, py, and pz.
As the principal quantum number increases (e.g., from 1s to 2s or from 2p to 3p), orbitals become larger and higher in energy. Therefore, a 2s orbital is larger and has more energy than a 1s orbital, and a 3p orbital is larger than a 2p orbital (Figure 1.9). Although the three p orbitals in a given shell (2px, 2py, and 2pz) differ in orientation—aligned along the x-, y-, and z-axes—they are identical in energy. Orbitals that have the same energy are called degenerate orbitals. Understanding the nature of orbitals is foundational to explaining how atoms bond, how molecules form, and how chemical reactions proceed—all key topics in the study of chemistry.
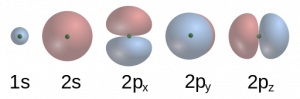
Figure 1.9: s and p Orbitals
Credits: https://commons.wikimedia.org/wiki/File:S-p-Orbitals.svg
1.9 Hybridization in a Carbon Atom
The 2s and 2p orbitals in a carbon atom mix or hybridize to form hybridized atomic orbitals. The concept of hybridization helps us understand how carbon forms four equivalent tetrahedral bonds and, how carbon atoms form double and triple bonds with other carbon and heteroatoms.
1.9.1 sp3 Hybridization
An s orbital and three p orbitals on an atom can combine, or hybridize, to form four equivalent atomic orbitals with tetrahedral orientation. These tetrahedrally oriented orbitals are called sp3 hybrid orbitals. Note that the superscript 3 in the name sp3 tells how many of each type of atomic orbital combine to form the hybrid (Figure 1.10).
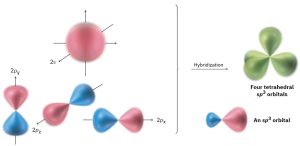
Figure 1.10: Four sp3 hybrid orbitals, oriented toward the corners of a regular tetrahedron, are formed by the combination of an s orbital and three p orbitals (red/blue). The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them adirectionality and allowing them to form strong bonds to other atoms.
The asymmetry of sp3 orbitals arises because, as noted previously, the two lobes of a p orbital have different algebraic signs, + and –, in the wave function. Thus, when a p orbital hybridizes with an s orbital, the positive p lobe adds to the s orbital but the negative p lobe subtracts from the s orbital. The resultant hybrid orbital is therefore unsymmetrical about the nucleus and is strongly oriented in one direction. When each of the four identical sp3 hybrid orbitals of a carbon atom overlaps with the 1s orbital of a hydrogen atom, four identical C–H bonds are formed and methane results. Each C–H bond in methane has a strength of 439 kJ/mol (105 kcal/mol) and a length of 109 pm (Figure 1.11). Because the four bonds have a specific geometry, we also can define a property called the bond angle. The angle formed by each H–C–H is 109.5°, the so-called tetrahedral angle. Methane thus has the structure shown in the following figure.
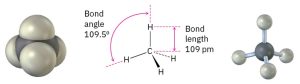
Figure 1.11: The structure of methane, showing its 109.5° bond angles and tetrahedral shape
We can picture the ethane molecule by imagining that the two carbon atoms bond to each other by head-on sigma (σ) overlap of an sp3 hybrid orbital from each (Figure 1.12). The remaining three sp3 hybrid orbitals on each carbon overlap with the 1s orbitals of three hydrogens to form the six C–H bonds. The C–H bonds in ethane are similar to those in methane, although a bit weaker: 421 kJ/mol (101 kcal/mol) for ethane versus 439 kJ/mol for methane. The C–C bond is 153 pm in length and has a strength of 377 kJ/mol (90 kcal/mol). All the bond angles of ethane are near, although not exactly at, the tetrahedral value of 109.5°.
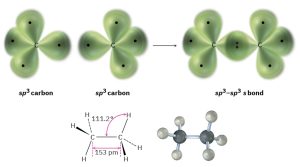
Figure 1.12: The carbon–carbon bond is formed by σ overlap of two sp3 hybrid orbitals.
Listen to this pencast on Sp3 Hybridization.
1.9.2 sp2 Hybridization
The 2s orbital combines with only two of the three available 2p orbitals. Three sp2 hybrid orbitals result, and one 2p orbital remains unchanged. Like sp3 hybrids, sp2 hybrid orbitals are unsymmetrical about the nucleus and are strongly oriented in a specific direction so they can form strong bonds. The three sp2 orbitals lie in a plane at angles of 120° to one another, with the remaining p orbital perpendicular to the sp2 plane (Figure 1.13).
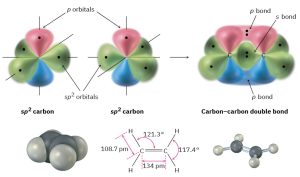
Figure 1.13: The structure of ethylene. One part of the double bond in ethylene results from σ (head-on) overlap of sp2 hybrid orbitals, and the other part results from π (sideways) overlap of unhybridized p orbitals (red/blue). The π bond has regions of electron density above and below a line drawn between nuclei.
Listen to this pencast on Sp2 Hybridization.
1.9.3 sp Hybridization
A carbon 2s orbital hybridizes with only a single p orbital. Two sp hybrid orbitals result, and two p orbitals remain unchanged. The two sp orbitals are oriented 180° apart on the right-left (x) axis, while the p orbitals are perpendicular on the up-down (y) axis and the in-out (z) axis.
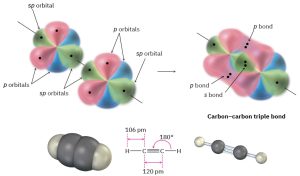
Figure 1.14: The structure of acetylene. The two carbon atoms are joined by one sp–sp σ bond and two p–p π bonds.
When two sp-hybridized carbon atoms approach each other, sp hybrid orbitals on each carbon overlap headon to form a strong sp–sp σ bond. At the same time, the pz orbitals from each carbon form a pz–pz π bond by sideways overlap, and the py orbitals overlap similarly to form a py–py π bond. The net effect is the sharing of six electrons and formation of a carbon–carbon triple bond. Each of the two remaining sp hybrid orbitals forms a σ bond with hydrogen to complete the acetylene molecule (Figure 1.14). Table 1.2 summarizes the three types of hybridization that we discussed in detail.
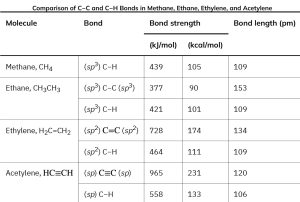
Table 1.2: Relationship between Bond Length, Bond Strength and Hybridization
To determine the hybridization of an atom in a molecule, we count the number of groups around the atom. The number of groups (atoms and non-bonded electron pairs) corresponds to the number of atomic orbitals that must be hybridized to form the hybrid orbitals (Table 1.3 and Figure 1.15).
| Number of Groups around the Central Atom | Hybridization | Number & types of orbitals | Bond Angle | Molecular Shape |
| 4 | sp3 | four sp3 hybrid orbitals | 109.5° | tetrahedral |
| 3 | sp2 | three sp2 hybrid orbitals + one p orbital | 120° | trigonal planar |
| 2 | sp | two sp hybrid orbitals + two p orbitals | 180° | linear |
Table 1.3: Determining Hybridization Based on the Number of Groups around the Central Atom
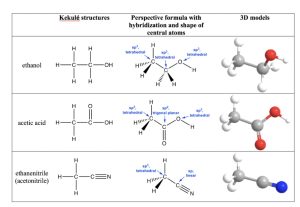
Figure 1.15: Examples of Hybridization in Organic Molecules
As the number of electrons between two nuclei increases, bonds become shorter and stronger. Triple bonds are shorter and stronger than double bonds, which are shorter and stronger than single bonds.
| Type of Bond | Bond Length A° | Bond Dissociation Energy (Kj/mol) |
| C-C (single bond) | 1.543 | 346 |
| C=C (double bond) | 1.483 | 602 |
| C≡C (triple bond) | 1.378 | 835 |
Table 1.4: Bond Length and Bond Dissociation Energies for Single, Double and Triple Bonds
Source: Data from J. E. Huheey, E. A. Keiter, and R. L. Keiter, Inorganic Chemistry, 4th ed. (1993); John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, “You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format.”
Examples
Pyridoxal phosphate, a close relative of vitamin B6, is involved in a large number of metabolic reactions. What is the hybridization and the bond angle for each nonterminal atom?
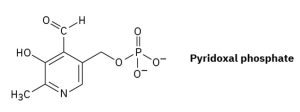
Answer:
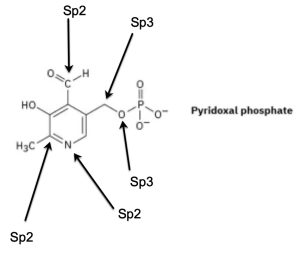
Key Takeaways: Hybridization Theory
- Hybridization theory explain bonding of carbon in organic molecules.
- There are three types of hybridizations for carbon: sp3, sp2 and sp.
- sp3 hybridization explains bonding of carbon in saturated compounds; sp2 hybridization explains bonding of carbon in unsaturated double bonded compounds while sp hybridization explains bonding of carbon in unsaturated triple bonded compounds.
- Hybridization explains both the structure and reactivity of organic compounds.
Exercise: Check your knowledge in hybridization theory
1.10 Drawing Three Dimensional Structures
In a three dimensional structure of a tetrahedral organic compound, there are three types of lines – a solid line, a solid wedge and a broken wedge. A solid line is used to indicate bond in the plane. A wedge is used to indicate a bond in front of the plane. A dashed line is used to indicate a bond behind the plane (Figure 1.16). The molecule can be turned in many different ways, generating many equivalent representations.
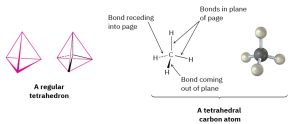
Figure 1.16: A representation of van’t Hoff’s tetrahedral carbon atom. The solid lines represent bonds in the plane of the paper, the heavy wedged line represents a bond coming out of the plane of the page toward the viewer, and the dashed line represents a bond going back behind the plane of the page away from the viewer.
We do not need to draw molecules in three dimensional manner all the time. We have choices depending on our needs.
There are three other types of structural formulas used in organic chemistry.
- Expanded Formulas
- Condensed Formulas
- Line-Angle Formulas
Expanded drawings are very useful for a beginning organic chemistry student. In this structure, all bond connections are clearly shown. Lone pairs are not drawn. Although cumbersome and time-consuming, these are useful structural drawings to represent isomers.
In a condensed structure, all bonds are drawn, but single bond lines are omitted. Atoms are usually drawn next to the atoms to which they are bonded. Parentheses are used around similar groups bonded to the same atom. Lone pairs are not drawn. Sometimes, multiple bonds are also not drawn.
Challenges: Interpreting parentheses, double bonds, triple bonds, branches and how these are all attached.
Line-Angle Formulas (sometimes referred to as skeletal drawings): Skeletal structures are commonly used to represent organic compounds, in particular for cyclic compounds, because of their ease of drawing. This drawing assumes that there is a carbon atom at the junction of any two lines or at the end of any line. Hydrogen atoms are always assumed and therefore omitted in the structure. The number of missing hydrogen atoms can be determined by the tetravalency of carbon. Heteroatoms and hydrogen atoms directly bonded to them are drawn and are never assumed. This by far, is the most popular form of drawing organic structures, in particular, for cyclic compounds. Here is a summary of the main points:
- Each vertex represents a carbon atom
- C-H bonds are omitted. The number of hydrogen atoms is determined by subtracting the drawn bonds from four.
- Heteroatoms must be specified, and O-H and N-H bonds are not omitted. These must be clearly drawn.
Examples
Quetiapine, marketed as Seroquel, is a heavily prescribed antipsychotic drug used in the treatment of schizophrenia and bipolar disorder. Convert the following representation into a skeletal structure, and give the molecular formula of quetiapine.
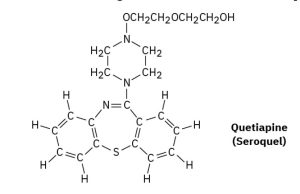
Answer:
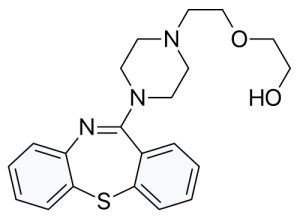
Molecular Formula: C21H25N3O2S
Exercises: Drawing Condensed Structures
1.11 Isomers
Compounds that have the same molecular formula but different arrangements are known as isomers. There are many types of isomerism. Structural isomers are those that differ in the arrangement of atoms.
Examples: Structural Isomers
Draw the indicated number of structural isomers for each of the following molecular structures.
Examples
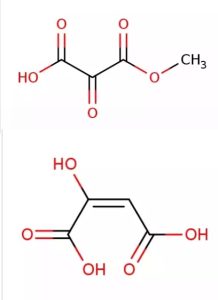
Oxaloacetic acid, an important intermediate in food metabolism, has the formula C4H4O5. Propose two possible structures.
Answer:
Examples
The following model is a representation of citric acid, the key substance in the so-called citric acid cycle, by which food molecules are metabolized in the body. Only the connections between atoms are shown; multiple bonds are not indicated. Complete the structure by indicating the positions of multiple bonds and lone-pair electrons (black = C, red = O, gray = H).
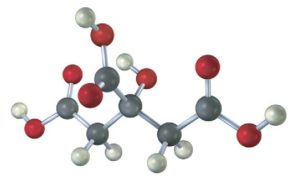
Answer:
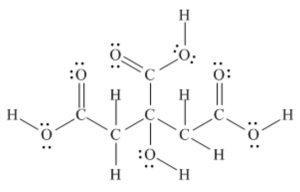
1.12 Theory of Resonance
Resonance is a concept that describes the delocalization of electrons within molecules or ions. Resonance structures are used when a single Lewis structure cannot fully describe the bonding that occurs within a molecule or ion (Figure 1.17). It involves constructing multiple Lewis structures that, when combined, represent the full electronic structure of the molecule or ion – the Resonance Hybrid. In general, molecules or ions with multiple resonance structures will be more stable than one with fewer. Some resonance structures contribute more to the stability of the molecule or ion than others – formal charges aid in making this determination. Resonance stabilization is a major determining factor in the outcome of many reactions.
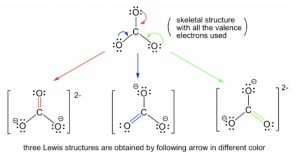
Figure 1.17: Carbonate Resonance Structures
Arrows are used for drawing resonance structures with ease. It is important to remember that the resonance hybrid is the most accurate representation and structures do not flip back and forth between individual resonance structures. Watch this video for an introduction to resonance structures. This sets the stage for drawing curved arrows and generating resonance structures from a given structure.
The way to use curved arrows to show electron transfer is also called arrow pushing, and it is a very important fundamental skill you need to master in organic chemistry. To construct “new” resonance structures, arrows have to be shown in the “original” structure.
The double bond can be built between the central C and any of the terminal O’s to generate three structures, and they all look “the same”. However, they are not really identical (or the same), they are just equivalent. Each structure is called a resonance structure, and they can be connected by the double-headed resonance arrow. There are three equivalent resonance structures for CO32-, and the actual structure of CO32-is a hybrid of the three resonance contributors (Figure 1.18).
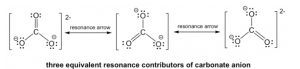
Figure 1.18: Three Equivalent Resonance Structures
Since the resonance structures are equivalent, they are all in the same level of energy and have the same stability, so they make the same contributions to the actual structure of CO32-. This is supported by experimental evidence showing that all the carbon-oxygen bonds in CO32- are the same bond length, which is longer than a regular double bond but shorter than a single bond. As a result of the resonance structures, the two negative charges in CO32- are not localized on any oxygen atoms, but are spread evenly among all three oxygen atoms, and this is called charge delocalization. Because of charge delocalization, each oxygen atom has two-thirds of a full negative charge. Charge delocalization helps stabilize the whole species. The stability a species gains from having charge delocalization through resonance contributors is called the resonance stabilization effect. The greater the number of resonance contributors, the greater the resonance stabilization effect and the more stable the species is.
The actual structure of the carbonate anion is a combination of all three equivalent resonance structures, which can be called a hybrid (Figure 1.19). What does the actual structure look like, and can we draw one structure on paper to show the actual structure? The actual structure can not be shown with a conventional Lewis structure because the regular Lewis structures do not include partial charges, and there are two-thirds of a full negative charge on each oxygen atom in CO32-. An attempt to show the hybrid structure can be done by using dashed lines to show that the bond between carbon and oxygen is somewhere between a single and double bond, and each oxygen atom has partial charges.
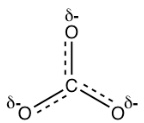
Figure 1.19: Resonance Hybrid of Carbonate Ion
The delocalized charges can also be represented by the calculated electrostatic potential map of the electron density in the CO32- anion. In an electrostatic potential map, regions with different charges are shown in different colours. More specifically, colours trending towards red mean higher negative charges, while colours trending toward blue mean more positive charges (the colour system generated by different types of software might not be the same, but they will follow the same trend). In the electrostatic potential map of the carbonate anion below, the same shade of red of all three oxygen atoms indicates the equal charge distribution at the three oxygen atoms (Figure 1.20).
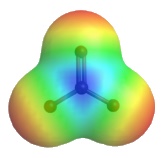
Figure 1.20: Electrostatic Potential Map of Carbonate Ion
There are several rules that must be followed when drawing curved arrows for resonance structures:
Rules for Drawing Resonance Structures:
- Only electrons move!! Nuclei (atoms) and single bonds do not move.
- Second-row elements do not exceed octet electrons.
- Resonance involves electrons in lone pairs and π bonds.
- All resonance structures must be valid Lewis structures (ions allowed).
- Track all lone pairs and formal charges.
- Arrow pushing are used to interconvert resonance structures.
- Double headed arrows are used to separate resonance structures.
- Lower energy resonance structures contribute more to the resonance hybrid.
Recognizing common patterns greatly help with drawing correct resonance structures. The following are five patterns commonly encountered with organic structures (Figure 1.21).
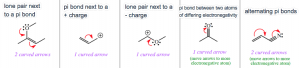
Figure 1.21: Common Resonance Patterns
Credits: https://www.oercommons.org/courses/homeunc-system-organic-chemistry-digital-course
Some useful resources…..
This document illustrates the correct method of drawing resonance structures.
This video explains the commonly observed patterns in resonance.
Common errors in drawing resonance structures:
- σ bond is moved.
- An Atom is moved.
- There are more than eight electrons located around C, N or O.
- Arrows are not shown in the proper way.
- Electron pairs are moved too far away; they should only be moved to the next position/atom.
Key Takeaways: Resonance Theory
- Resonance structures are multiple correct Lewis structures drawn for a particular molecule or ion.
- All rules for Lewis structures must be followed while drawing resonance structures. Formal charges must be correctly placed when it is other than 0.
- Move only pi electrons and lone pairs.
- Always move electrons from electron rich site to electron poor site.
- Never disobey the law of conservation of matter – do not create or destroy electrons. Only move them!
Exercises: Draw resonance structures for each given Lewis structure.
Among the various resonance structures, the most contributing or significant resonance structure can be determined by applying the following rules:
Rule 1: The most significant resonance contributor has the least number of atoms with formal charges.
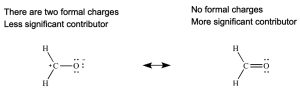
Rule 2: The most significant resonance contributor has the greatest number of full octets (or if applicable, expanded octets).
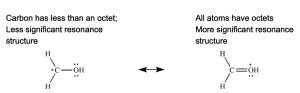
Rule 3: If formal charges cannot be avoided, the most significant resonance contributor has the negative formal charges on the most electronegative atoms, and the positive formal charges on the least electronegative atoms.

Rule 4: The most significant resonance contributor has the greatest number of covalent bonds.
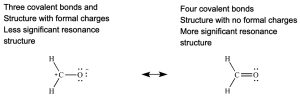
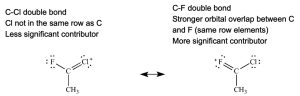
Rule 5: If a pi bond is present, the most significant resonance contributor has this pi bond between atoms of the same row of the periodic table (usually carbon pi bonded to boron, carbon, nitrogen, oxygen, or fluorine).
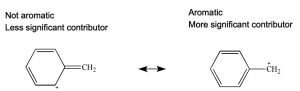
Rule 6: Aromatic resonance contributors are more significant than resonance contributors that are not aromatic.
Examples
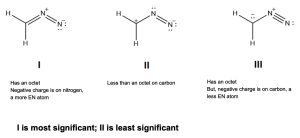
Identify the most and the least contributing resonance structure and state your reason.
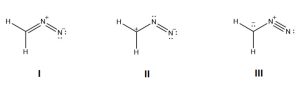
Answer:
Key Takeaways for Chapter 1
- The study of carbon compounds is known as organic chemistry.
- Organic compounds are formed by covalent bonding.
- Difference in electronegativity between atoms in shared bonds leads to polar covalent bonds.
- VSEPR theory predicts the shapes of molecules. The three main shapes encountered in organic compounds are tetrahedral, planar and linear.
- Hybridization theory explain bonding modes of carbon in single, double and triple bonded compounds.
- Condensed and skeletal structures are used to represent organic compounds.
of an element is equal to the # of protons. For example, atomic number of carbon is 6. This implies that there are 6 protons in the nucleus of carbon.
of an element is the weighted average of all isotopes of the element. For example, the atomic mass of carbon is 12.011 amu
of an element is the sum of protons and neutrons For example, carbon has 6 protons and 6 neutrons. The mass number of carbon is 12.
are atoms of an element with same atomic number but different mass number. Examples of isotopes are 12C6, 13C6 and 14C6. In each case, carbon has the same atomic number but different mass number. In other words, each isotope of carbon has the same number of protons but different number of neutrons.
Valence electrons are the electrons in the outermost shell. They impart special properties to the element. Electrons in the inner shells are known as the core electrons. The group number to which the element belongs will indicate the # of valence electrons in that particular element. For example, nitrogen belongs to group V. Therefore, there are 5 valence electrons in a nitrogen atom.
Provides a description of the arrangement of electrons in the orbitals of an atom.
For example, ground state electron configuration of carbon is 1s2 2s2 2p2. Valence electron configuration is 2s2 2p2.
A symbol used to indicate 'partial'
Indicates bonding modes where the atoms do not carry a charge.
Valence Shell Electron Pair Repulsion Theory
shape of molecule determined by both bonding as well as well as nonbonding pairs of electrons
shape of molecule determined by only bonding pairs of electrons
compounds that contain rings
atoms other than carbon and hydrogen are referred to as heteroatoms in organic chemistry.
Examples of heteroatoms are S, N, P

