3 Acids and Bases
Chapter 3 Learning Objectives
- Define an acid and a base based on Arrhenius definitions.
- Be able to define an acid and a base based on Bronsted-Lowry definition.
- Identifying factors such as electronegativity and atom size, charge, resonance, inductive effects, hybridization and their impact on acidity and basicity
- Relate pKa to acidity and acidity trends
- Predict the outcome of acid-base reactions based on pKa values.
- Be able to define an acid and a base based on Lewis theory.
- Be able to identify an electrophile and a nucleophile in an acid-base reaction.
- Be able to predict the product of a Lewis acid-base reaction.
- Be able to draw electron pushing curved arrows for a given reaction.
3.1 Introduction
Acids and bases serve many roles such as catalysts, reactants, or products in an organic reaction and as such, identifying and utilizing acids and bases correctly is critical in understanding organic chemistry reactions. This module focuses on the importance of acids and bases in organic chemistry reactions by focusing on the following skills: understanding the Lewis definition of acids, correctly identifying and differentiating between the types of acids and bases, identifying structural factors that determine relative strengths of acids and bases, using relative strengths of acids and bases to determine the position of equilibrium and to correctly predict the products of acid-base reactions.
Watch the following CrashCourse video for the key points of acidity related to organic chemistry.
3.2 Arrhenius Acids and Bases
Additional Resources: Arrhenius Acids and Bases
- Arrhenius acids and bases by LibreTexts is available under a CC BY-NC-SA license.
- Arrhenius acid-base reactions by LibreTexts is available under a CC BY-NC-SA license
- Read and watch this video on Arrhenius acids and bases.
3.3 Bronsted-Lowry Acids and Bases
A Brønsted–Lowry acid is a substance that donates a hydrogen ion, H+, and a Brønsted–Lowry base is a substance that accepts a hydrogen ion. (The name proton is often used as a synonym for H+ because loss of the valence electron from a neutral hydrogen atom leaves only the hydrogen nucleus—a proton.) When gaseous hydrogen chloride dissolves in water, for example, a polar HCl molecule acts as an acid and donates a proton, while a water molecule acts as a base and accepts the proton, yielding chloride anion (Cl–) and hydronium cation (H3O+). This and other acid–base reactions are reversible, so we’ll write them with double, forward and backward arrows (Scheme 3.1).
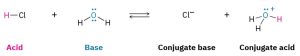
Scheme 3.1: Reaction Between An Acid And A Base
Chloride ion, the product that results when the acid HCl loses a proton, is called the conjugate base of the acid, and hydronium ion, the product that results when the base H2O gains a proton, is called the conjugate acid of the base. Other common mineral acids such as H2SO4 and HNO3 behave similarly, as do organic acids such as acetic acid, CH3CO2H.
Additional Resources: Bronsted-Lowry Acids and Bases
- Read and watch this video on Bronsted Lowry acids and bases.
- Brønsted-Lowry concept of acids and bases by LibreTexts is available under a CC BY-NC-SA license.
- Bronsted-Lowry acids and bases may provide additional information in Introductory Chemistry, 1st Canadian Edition by David W. Ball and Jessie A. Kay is available under CC BY-NC-SA license.
Additional Resources: Conjugate Acid-Base Pairs
Read and watch this resource on conjugate acid-base pair.
For the conjugate acid-base pair, the stronger the acid, the weaker the conjugate base is, and vice versa.
Exercise 3.1: Check your knowledge in conjugate acid-base theory.
Key Takeaways: Bronsted-Lowry Acids and Bases
-
A Brønsted-Lowry acid is any species that is capable of donating a proton,
-
A Brønsted-Lowry base is any species that is capable of accepting a proton, H+
-
Some molecules can act as both a Brønsted-Lowry acid and a Brønsted-Lowry base.
-
The conjugate base of a Brønsted-Lowry acid is the species formed after an acid donates a proton. The conjugate acid of a Brønsted-Lowry base is the species formed after a base accepts a proton.
-
The two species in a conjugate acid-base pair have the same molecular formula except the acid has an extra H+
3.4 Factors That Affect Acidity And Basicity
3.4.1 Electronegativity
The element effect is about the individual atom that connects with the hydrogen (keep in mind that acidity is about the ability to donate certain hydrogen). A clear trend in the acidity of these compounds is that the acidity increases for the elements from left to right along the second row of the periodic table, C to N, and then to O. This is consistent with the increasing trend of EN along the period from left to right. The connection between EN and acidity can be explained as the atom with a higher EN being better able to accommodate the negative charge of the conjugate base, thereby stabilizing the conjugate base in a better way. Therefore, the more stable the conjugate base, the weaker the conjugate base is, and the stronger the acid is. For the discussion in this section, the trend in the stability (or basicity) of the conjugate bases often helps explain the trend of the acidity.
When moving vertically within a given group on the periodic table, the trend is that acidity increases from top to bottom. This can be illustrated with the haloacids HX and halides as shown below: the acidity of HX increases from top to bottom, and the basicity of the conjugate bases X– decreases from top to bottom.
3.4.2 Resonance
The resonance effect accounts for the acidity difference between ethanol and acetic acid. For both ethanol and acetic acid, the hydrogen is bonded with the oxygen atom, so there is no element effect that matters. However, the pKa values (and the acidity) of ethanol and acetic acid are very different. What makes a carboxylic acid so much more acidic than an alcohol? As stated before, we begin by considering the stability of the conjugate bases, remembering that a more stable (weaker) conjugate base corresponds to a stronger acid.
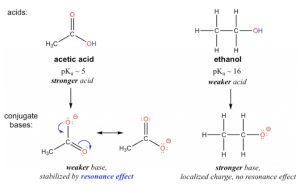
Figure 3.1: Resonance Effect
3.4.3 Inductive Effect
The presence of the chlorine atoms clearly increases the acidity of the carboxylic acid group, and the trend here apparently can not be explained by the element effect. The resonance effect does not apply here either, because no additional resonance contributors can be drawn for the chlorinated molecules. Rather, the explanation for this phenomenon involves something called the inductive effect. A chlorine atom is more electronegative than hydrogen and is thus able to ‘induce’ or ‘pull’ electron density towards itself via σ bonds in between, and therefore it helps spread out the electron density of the conjugate base, the carboxylate, and stabilize it. The chlorine substituent can be referred to as an electron-withdrawing group because of the inductive effect (Figure ). Greater the electronegativity of the halogen atom, stronger is the inductive effect (Figure 3.2 ).
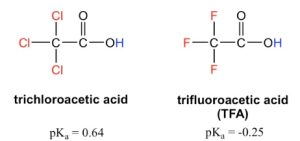
Figure 3.2: Relative Inductive Effect
Inductive effect is a distance dependent effect. The farther the halogen atom is from the acidic hydrogen, weaker is the inductive effect (Figure 3.3).
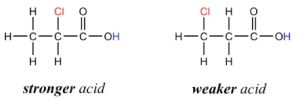
Figure 3.3: Effect of Distance on Inductive Effect
The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The inductive effect is additive; more chlorine atoms have an overall stronger effect, which explains the increasing acidity from mono, to di-, to tri-chlorinated acetic acid (Figure 3.4).
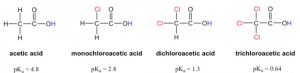
Figure 3.4: Presence of Inductive Effect
3.4.4 Hybridization Effect
The key difference between the conjugate base anions is the hybridization of the carbon atom, which is sp3, sp2 and sp for alkane, alkene and alkyne, respectively. Different hybridizations lead to different s character, which is the percent of s orbitals out of the total number of orbitals. The sp3 hybridization means 25% s character (one s and three p orbitals, so s character is 1/4 = 25%), sp2 hybridization has 33.3% s character, and the number is 50% for sp hybridization. Electrons of 2s orbitals are in a lower energy level than those of 2p orbitals because 2s is much closer to the nucleus. So, for an anion with more s character, the electrons are closer to the nucleus and experience stronger attraction; therefore, the anion has lower energy and is more stable (Figure 3.5).
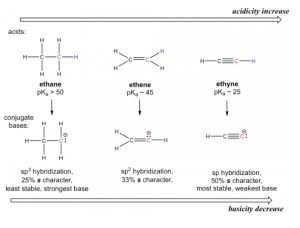
Figure 3.5: Hybridization Effect
- Watch the following videos that describe how acidity is related to structure. Video 1 Video 2 Video 3 Video 4 Video 5
- Watch this video that relates Ka and pKa to acidity.
- Read this article that explain Ka and pKa; how this related to acid strength.
- Read this page that lists a summary of factors that affect acid strength.
Exercise 3.2: Test your knowledge on the factors that affect acid strength.
Exercise 3.3:
For the following pairs of species, use your understanding of the factors that affect acidity to identify which species in each pair is the acid and which is the base. Then determine the conjugate acid and conjugate base formed in the reaction. Show the arrow-pushing mechanism of product formation.
3.5 Predicting the Position of Equilibrium In Acid-Base Reactions
Now, that you have mastered the factors that affect acidity and basicity, you are ready to learn how to predict the position of the equilibrium in an acid-base reaction based on pKa values. H+ will always go from the stronger acid to the stronger base. That is, an acid will donate a proton to the conjugate base of a weaker acid, and the conjugate base of a weaker acid will remove a proton from a stronger acid.
Since water (pKa = 15.74) is a weaker acid than acetic acid (pKa = 4.76), for example, hydroxide ion holds a proton more tightly than acetate ion does. Hydroxide ion will therefore react to a large extent with acetic acid, CH3CO2H, to yield acetate ion and H2O (Scheme 3.2).
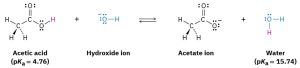
Scheme 3.2: Position of Equilibrium
A general rule for acid-base reactions: Acid-base reactions always favor the formation of the weaker acid and the weaker base. This is because the equilibrium always favors the formation of more stable products, and weaker acids and bases are more stable than stronger ones.
Additional Resources:
- Read about how pKa values can be used to predict the outcome of acid-base reactions.
- This is another article that clearly describes the steps to determine the position of the equilibrium in acid-base reactions.
Exercise 3.4: In each reaction, identify the stronger acid when comparing acid to conjugate acid. Then, determine whether equilibrium lies to the left or to the right in each reaction.
3.6 Lewis Acids and Bases
Although Bronsted theory is still widely used, it is restricted to compounds that can donate or accept protons. In 1923, G.N. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases. His theory gave a more generalized explanation of acids and bases based on electron pairs. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions.
A Lewis acid is an electron pair acceptor while a Lewis base is an electron pair donor. A Lewis acid-base reaction occurs when a base donates a pair of electrons to an acid. A Lewis acid-base adduct, a compound that contains a coordinate covalent bond between the Lewis acid and the Lewis base, is formed. Lewis acids are also known as electrophiles (electron loving) and Lewis bases are known as nucleophiles (nucleus loving or positive charge loving). Lewis acids will have vacant orbitals to accept electrons. Lewis bases will have lone pairs or pi bonds to donate.
There are different chemical species that can act as Lewis acids.
- All cations are Lewis acids since they are able to accept electrons. (e.g., Cu2+, Fe2+, Fe3+)
- An atom, ion, or molecule with an incomplete octet of electrons can act as an Lewis acid (e.g., BF3, AlF3). These are commonly used Lewis acids in several organic reations.
- Molecules where the central atom can have more than 8 valence shell electrons can be electron acceptors, and thus are classified as Lewis acids (e.g., SiBr4, PF5).
- Molecules that have multiple bonds between two atoms of different electronegativities (e.g., CO2, SO2)
Lewis bases are species that contain lone pairs such as NH3 or H2O or pi bonds such as CH2=CH2.
Some examples of acid-base reactions depicting the Lewis acid-base concept are shown here (Scheme 3.3):
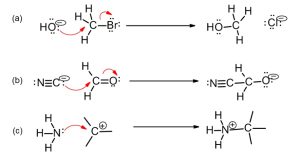
Scheme 3.3: Examples of Lewis Acid-Base Reactions
In reaction (a), HO– ion is the nucleophile. Oxygen is the nucleophilic site and transfers a pair of electrons to carbon of the other molecule. Carbon carries a partial positive charge due to the polarity of the C-Br bond. Remember to cleave the C-Br bond or you will end up with more than an octet number of electrons in carbon’s valence shell. The overall charge of both the reactants and the products remains the same, -1.
In reaction (b), the cyanide ion is the nucleophile and carbon is the nucleophilic site. It transfers a pair of electrons to the carbonyl carbon atom (this atom carries a partial positive charge due to the polarity of the C-O bond). In this case, the pi bond is broken and polarized towards the oxygen atom. The overall charge of the reactants and products is constant, -1.
In reaction (c), NH3 is the nucleophile and nitrogen is the nucleophilic site. The electron pair on the nitrogen atom is donated to the empty p-orbital of the positively charged carbon atom. This carbon atom is the electrophilic site. The nitrogen atom in the product carries a formal charge of +1.
Additional Resources: Lewis Acids and Bases
- A very detailed analysis of the Lewis acid-base theory can be found here.
- Lewis acids and bases by LibreTexts is available under a CC BY-NC-SA license.
3.7 Drawing Curved Arrows
For a new organic chemistry student, drawing curved arrows can seem a complex process. However, the underlying principle is fairly simple, shows the movement of electrons from an electron rich site to an electron poor site. The following rules also help you draw the arrows correctly.

Other key points to note are:
- The electronegativity of carbon atoms varies as Csp3 < Csp2 < Csp. Do not move electrons from atoms of greater electronegtivity towards those of lower electronegativity.
- Second row elements cannot have more than octet in their valence shells.
- Watch this CrashCourse video on “Nucleophiles and Electrophiles”.
Exercise 3.5: Test your knowledge in the basics of reaction mechanisms
Exercise 3.6: Test yourself: Using the following structures, determine the Lewis acid-base reaction that will occur. Label the Lewis base as the nucleophile and the Lewis acid as the electrophile. Show product formation by using an arrow-pushing mechanism.
Exercise 3.7:
In pharmaceutical synthesis, the reaction between a carbonyl compound (such as an aldehyde or ketone) and a nucleophile often requires the presence of a Lewis acid catalyst like BF₃ (boron trifluoride) to proceed efficiently. For example, in the Friedel-Crafts acylation reaction, BF₃ is used to activate the carbonyl group of an acid chloride, making it more electrophilic.
Using Lewis acid-base theory, explain why BF₃ acts as a Lewis acid in this reaction. Identify the Lewis base involved and describe how the interaction facilitates the overall organic transformation.
Key Takeaways: Lewis Acid-Base Theory
- Lewis acids are electron pair acceptors.
- Lewis bases are electron pair donors.
- Lewis acids are also known as electrophiles.
- Lewis bases are also known as nucleophiles.
- Electrons are transferred from the HOMO of the nucleophile to the LUMO of the electrophile.
- A Lewis acid-base adduct is formed when a reaction occurs between a Lewis acid and a Lewis base.
ability of an atom to attract a shared pair of electrons

