17 Nomenclature and Physical Properties of Alkanes and Cycloalkanes
Chapter 17 Learning Objectives
-
Define and classify alkanes (acyclic saturated hydrocarbons) and cycloalkanes (cyclic saturated hydrocarbons).
-
Draw and name alkanes and cycloalkanes using IUPAC nomenclature, including branched and substituted systems.
-
Identify and differentiate between constitutional isomers of alkanes and cycloalkanes.
-
Predict physical properties (boiling point, melting point, solubility) based on molecular structure and intermolecular forces.
-
Recognize and explain the trends in physical properties across straight-chain and branched alkanes and cycloalkanes.
17.1 Introduction
Hydrocarbons are made up of carbon and hydrogen atoms. Saturated hydrocarbons contain only C-C and C-H single bonds. Unsaturated hydrocarbons contain multiple bonds (such as double and triple bonds) between carbon atoms. Alkanes are saturated hydrocarbons. All C atoms in an alkane are surrounded by four groups, making them sp3 hybridized and tetrahedral, and all bond angles are 109.5°. General formula of alkanes is CnH2n+2.
17.2 Nomenclature of Alkanes
In this chapter, we will learn about nomenclature, structure and reactivity of alkanes and cycloalkanes. In the early 19th century, organic compounds’ names were selected by their discoverer. These are referred to as common names and many of them such as morphine are still in use today. As the library of organic compounds grew, it became essential for a systematic method of naming compounds to be established. In 1892, a group of chemists, now referred to as the International Union of Pure and Applied Chemistry (IUPAC) met in Switzerland and developed a system of organic nomenclature called the Geneva rules, now referred to as IUPAC nomenclature. Both IUPAC/systematic and common names will be discussed. The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning which chemical compound the name refers to: each chemical name should refer to a single substance.
Additional Resources
- This video is an introduction to IUPAC nomenclature.
- Watch this video on the basics of organic nomenclature.
- A step by step instructional guide on naming alkanes can be found here.
- How to name straight chain alkanes?
- How to name branched chain alkanes?
Exercise 17.1: Name the given alkanes using IUPAC rules.
Exercise 17.2: Draw the structure of the alkane for each given name.
Check out this resource for naming cycloalkanes.
17.3 Physical Properties of Alkanes
Alkanes show regular increases in both boiling point and melting point as molecular weight increases an effect due to the presence of weak dispersion forces between molecules. Only when sufficient energy is applied to overcome these forces does the solid melt or liquid boil. As you might expect, dispersion forces increase as molecular size increases, accounting for the higher melting and boiling points of larger alkanes.
Another effect seen in alkanes is that increased branching lowers an alkane’s boiling point. Thus, pentane has no branches and boils at 36.1 °C, isopentane (2-methylbutane) has one branch and boils at 27.85 °C, and neopentane (2,2-dimethylpropane) has two branches and boils at 9.5 °C. Similarly, octane boils at 125.7 °C, whereas isooctane (2,2,4-trimethylpentane) boils at 99.3 °C. Branched-chain alkanes are lower-boiling because they are more nearly spherical than straight-chain alkanes, have smaller surface areas, and consequently have smaller dispersion forces.
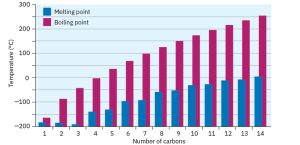
Figure 17.1 A plot of melting and boiling points versus number of carbon atoms for the C1–C14 straight-chain alkanes. There is a regular increase with molecular size.
The following article has been modified under the Creative Commons license. The original article was published in The Conversation. The authors of the original article are Professor of Astrophysics, University of Lancashire and Senior Lecturer in Astronomy, University of Lancashire
Nasa’s Curiosity Mars rover has detected the largest organic (carbon-containing) molecules ever found on the red planet. The discovery is one of the most significant findings in the search for evidence of past life on Mars. This is because, on Earth at least, relatively complex, long-chain carbon molecules are involved in biology. These molecules could actually be fragments of fatty acids, which are found in, for example, the membranes surrounding biological cells. The organic molecules found by Curiosity consist of carbon atoms linked in long chains, with other elements bonded to them, like hydrogen and oxygen. Among the molecules were decane, which has 10 carbon atoms and 22 hydrogen atoms, and dodecane, with 12 carbons and 26 hydrogen atoms. These are known as alkanes, which fall under the umbrella of the chemical compounds known as hydrocarbons.
Exercise 17.3: Name the cycloalkanes using IUPAC rules.
Exercise 17.4: Draw cycloalkanes for each given name.

