19 Conformational Analysis of Cycloalkanes
Chapter 19 Learning Objectives
- Describe ring strain in small cycloalkanes and relate it to angle strain, torsional strain, and steric strain.
- Be able to explain why cyclohexane is the most stable ring among cycloalkanes.
- Draw and compare the chair conformations of cyclohexane.
- Assign axial and equatorial positions and analyze their effects on the stability of monosubstituted and disubstituted cyclohexanes.
- Be able to draw both chair conformations of disubstituted cyclohexanes and identify the most stable conformation.
- Be able to poly substituted cyclohexane chair conformations and identify the most stable conformation.
19.1 Stability of Cycloalkanes
What causes the difference in stability or the strain in small cycloalkanes?
Chemists in the late 1800s knew that cyclic molecules existed, but the limitations on ring size were unclear. Although numerous compounds containing five-membered and six-membered rings were known, smaller and larger ring sizes had not been prepared, despite many attempts. A theoretical interpretation of this observation was proposed in 1885 by Adolf von Baeyer, who suggested that small and large rings might be unstable due to angle strain—the strain induced in a molecule when bond angles are forced to deviate from the ideal 109° tetrahedral value. Baeyer based his suggestion on the simple geometric notion that a three-membered ring (cyclopropane) should be an equilateral triangle with bond angles of 60° rather than 109°, a four-membered ring (cyclobutane) should be a square with bond angles of 90°, a five-membered ring should be a regular pentagon with bond angles of 108°, and so on. Continuing this argument, large rings should be strained by having bond angles that are much greater than 109°.
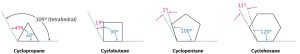
The data in the following figure shows that Baeyer’s theory is only partially correct. Cyclopropane and cyclobutane are indeed strained, just as predicted, but cyclopentane is more strained than predicted, and cyclohexane is strainfree. Cycloalkanes of intermediate size have only modest strain, and rings of 14 carbons or more are strain-free.
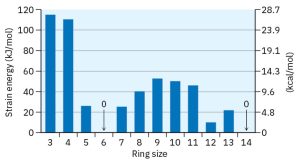
Figure 19.1 Comparison of Strain Energy Versus Ring Size
Why is Baeyer’s theory wrong?
Baeyer’s theory is wrong for the simple reason that he assumed all cycloalkanes to be flat. In fact, most cycloalkanes are not flat; instead, they adopt puckered three-dimensional conformations that allow bond angles to be nearly tetrahedral. As a result, angle strain occurs only in three- and four-membered rings, which have little flexibility. For most ring sizes, particularly the medium-ring (C7–C11) cycloalkanes, torsional strain caused by H⟷H eclipsing interactions at adjacent carbons and steric strain caused by the repulsion between non-bonded atoms that approach too closely are the most important factors.
There are three major types of strain that leads to instability of some cycloalkanes over others.
| Torsional Strain | arises due to repulsion in electrons in eclipsing bonds |
| Steric Strain | when bulky groups are in close proximity leading to unfavorable interactions between nuclei of atoms |
| Angle Strain | when the sp3 hybridized carbons in cycloalkanes do not have the expected ideal bond angle of 109.5o, causing an increase in the potential energy. |
19.2 Cyclopropane
Cyclopropane has angle strain where the angles are 600 between the carbon atoms instead of the theoretical prediction of 109.50. This large reduction in bond angles leads to poor overlap of Csp3-Csp3 hybrid orbitals. The bonds therefore are bent and popularly known as ‘bent banana bonds’. Cyclopropane also experiences severe torsional strain.
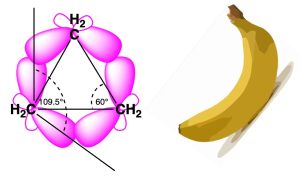
Figure 19.2 Bent Banana Bonds in Cyclopropane
19.3 Cyclobutane
Cyclobutane reduces some bond-eclipsing strain by folding (the out-of-plane dihedral angle is about 25º), but the total eclipsing and angle strain remains high.
Figure 19.3 Puckered Conformation of Cyclobutane
Cyclopentane has very little angle strain (the angles of a pentagon are 108º), but its eclipsing strain would be large (about 40 kJ/mol) if it remained planar. Consequently, the five-membered ring adopts non-planar puckered conformations whenever possible. This conformation is also known as ‘envelope’ conformation.
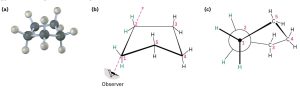
Figure 19.4 Envelope Conformation of Cyclopentane
A planar structure for cyclohexane is clearly impossible. The bond angles would necessarily be 120º, a large deviation from the ideal tetrahedral angle. Also, every carbon-hydrogen bond in such a structure would be eclipsed. The resulting angle and eclipsing strains would severely destabilize this structure. Therefore, the ring adopts a stable chair conformation.
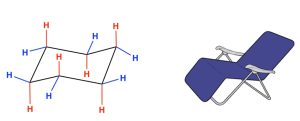
Figure 19.5 Chair Conformation of Cyclohexane
There are two types of hydrogen atoms, axial (red) and equatorial (blue). Review the following resources for additional information on cyclohexane chair conformations.
Additional Resources
- View this video that explain the chair and boat conformations of cyclohexane and how ring flip occurs.
- Here is a series of video tutorials that will bring you up to speed on:
Table 19.1 summarizes the isomers and conformations of 1,2; 1,3; and 1,4-disubstituted cyclohexanes.
| Substitution | Stereo | Ax/Eq? | Structure |
| 1,2-disubstituted | trans | e,e and a,a | 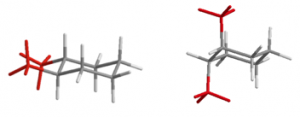 |
| cis | a,e | 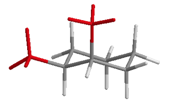 |
|
| 1,3-disubstituted | trans | a,e | 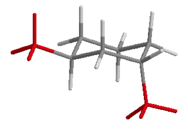 |
| cis | e,e and a,a | 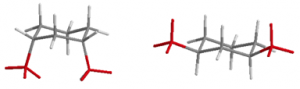 |
|
| 1,4-disubstituted | trans | e,e and a,a | 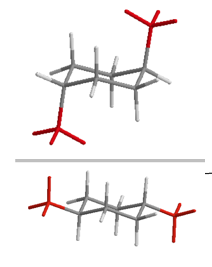 |
| cis | a,e | 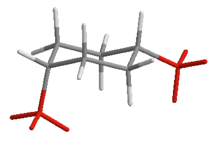 |
Table 19.1 Conformations of Disubstituted Cyclohexanes
Exercises 19.1: Cyclohexane Chair Conformations
Draw both chair conformations of cis-1-tert-butyl-4-methylcyclohexane. Identify the more stable conformation.
Draw both chair conformations of trans-1-tert-butyl-4-methylcyclohexane. Identify the more stable conformation.
Identify the most stable conformation between all conformations drawn.
Exercises 19.2: Conformations of Sugars
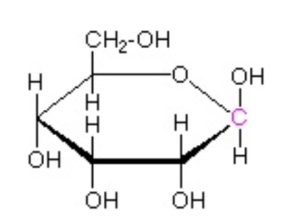
Draw the most stable chair conformation for D-Allopyranose (structure shown below).
Exercise 19.3: Steroid Conformation
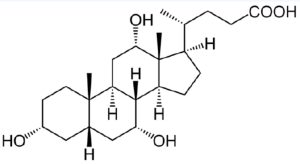
The following molecule is cholic acid. Draw it showing the chair conformations. Determine if the three fused bonds have a cis or trans configuration. Determine if the OH groups are axial or equatorial.
Exercises 19.4: Steroid Conformation
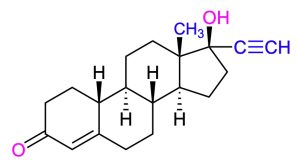
The following molecule is norethindrone. Draw it showing the chair conformations. Determine if the three fused bonds have a cis or trans configuration. Determine if the OH groups are axial or equatorial.
Key Takeaways: Cyclohexane Conformations
- Cyclohexane exists in the stable chair conformation. There are six axial and six equatorial bonds in cyclohexane.
- Ring flipping converts axial bonds to equatorial bonds and vice-versa.
- Substituents prefer to occupy equatorial position so that they do not experience destabilizing 1,3-diaxial interactions (steric strain). Larger the size of the substituent, the more it prefers the equatorial position.
- The cis isomer has two groups on the same side of the ring. The trans isomer has two groups on opposite sides of the ring.
Key Takeaways: Chapter Summary
You can view the chapter summary here.

