33 How to Interpret a Mass Spectrum?
Chapter 33 Learning Objectives
-
Interpret a mass spectrum to determine:
-
The molecular ion (M⁺) peak and its significance.
-
Fragmentation patterns and their relation to molecular structure.
-
Isotopic patterns for elements like Cl, Br, and S.
-
-
Calculate the molecular weight of an unknown compound using mass spectral data.
33.1 The Nitrogen Rule
The nitrogen rule of mass spectrometry says that a compound with an odd number of nitrogen atoms has an odd-numbered molecular weight. The logic behind the rule comes from the fact that nitrogen is trivalent, thus requiring an odd number of hydrogen atoms. The presence of nitrogen in a molecule is often detected simply by observing its mass spectrum. An odd-numbered molecular ion usually means that the unknown compound has one or three nitrogen atoms, and an even-numbered molecular ion usually means that a compound has either zero or two nitrogen atoms.
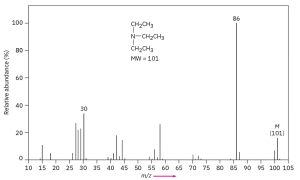
Mass Spectrum of Triethylamine
33.2 Presence of One Chlorine Atom
The fact that some elements have two common isotopes gives their mass spectra a distinctive appearance. Chlorine, for example, exists as two isotopes, 35Cl and 37Cl, in roughly a 3 : 1 ratio. In a sample of chloroethane, three out of four molecules contain a 35Cl atom and one out of four has a 37Cl atom. In the mass spectrum of chloroethane, we see the molecular ion (M) atm/z = 64 for ions that contain a 35Cl and another peak atm/z = 66, called the M + 2 peak, for ions containing a 37Cl. The ratio of the relative abundance of M:M +2 is about 3 : 1, a reflection of the isotopic abundances of chlorine.
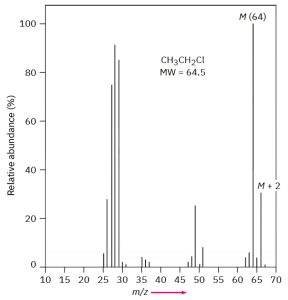
Figure XX: Mass Spectrum of Chloroethane
33.3 Presence of One Bromine Atom
In the case of bromine, the isotopic distribution is 50.7% 79Br and 49.3% 81Br. In the mass spectrum of 1-bromohexane (FIGURE 12.13) the molecular ion appears atm/z = 164 for 79Br-containing ions and the M + 2 peak is at m/z = 166 for 81Br-containing ions. The ions at m/z = 135 and 137 are informative as well. The two nearly equally large peaks tell us that the ions at thosem/z values still contain the bromine atom. The peak at m/z = 85, on the other hand, does not contain bromine because there is not a large peak at m/z = 87.
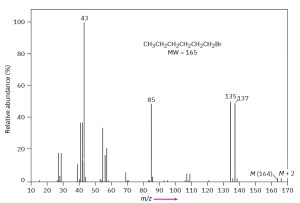
Mass Spectrum of 1-Bromohexane
Examples
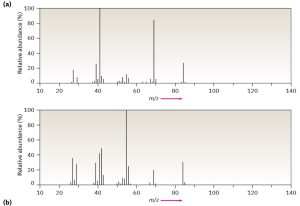
Assume that you have two unlabeled samples, one of methylcyclohexane and the other of ethylcyclopentane.
How could you use mass spectrometry to tell them apart?
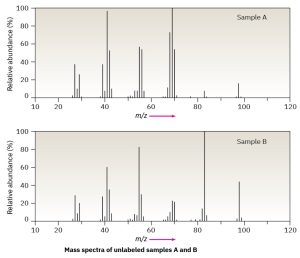
Answer:
Both mass spectra show molecular ions at M+ = 98, corresponding to C7H14, but they differ in their fragmentation patterns. Sample A has its base peak at m/z = 69, corresponding to the loss of a CH2CH3 group (29 mass units), but B has a rather small peak at m/z = 69. Sample B shows a base peak at m/z = 83, corresponding to the loss of a CH3 group (15 mass units), but sample A has only a small peak at m/z = 83. We can therefore be reasonably certain that A is ethylcyclopentane and B is methylcyclohexane.
Exercises 33.1: One spectrum is that of 2-methyl-2-pentene; the other is of 2-hexene. Which is which? Explain.