32 Principles of Mass Spectrometry
Chapter 32 Learning Objectives
- Describe the basic principles of mass spectrometry, including ionization, acceleration, deflection, and detection of ions.
- Describe the X- and Y-axis of a mass spectrum.
At its simplest, mass spectrometry (MS) is a technique for measuring the mass, and therefore the molecular weight (MW), of a molecule. In addition, it’s often possible to gain structural information about a molecule by measuring the masses of the fragments produced when molecules are broken apart.
A small amount of sample is vaporized into the ionization source, where it is bombarded by a stream of high-energy electrons. The energy of the electron beam can be varied but is commonly around 70 electron volts (eV), or 6700 kJ/mol. When a high energy electron strikes an organic molecule, it dislodges a valence electron from the molecule, producing a cation radical—cation because the molecule has lost an electron and now has a positive charge; radical because the molecule now has an odd number of electrons.
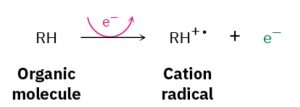
Scheme 32.1 Formation of Cation Radical Fragments
Electron bombardment transfers so much energy that most of the cation radicals fragment after formation. They break apart into smaller pieces, some of which retain the positive charge and some of which are neutral. The fragments then flow through a curved pipe in a strong magnetic field, which deflects them into different paths according to their mass-to-charge ratio (m/z). Neutral fragments are not deflected by the magnetic field and are lost on the walls of the pipe, but positively charged fragments are sorted by the mass spectrometer onto a detector, which records them as peaks at the various m/z ratios. Since the number of charges z on each ion is usually 1, the value ofm/z for each ion is simply its massm. Masses up to approximately 2500 atomic mass units (amu) can be analyzed by this type of instrument.
Click on various parts of a mass spectrometer to learn more.
The mass spectrum of a compound is typically presented as a bar graph, with masses (m/z values) on the x axis and intensity, or relative abundance of ions of a givenm/z striking the detector, on the y axis. The tallest peak, assigned an intensity of 100%, is called the base peak, and the peak that corresponds to the unfragmented cation radical is called the parent peak, or the molecular ion (M+, or simply M). The following figure shows the mass spectrum of propane.
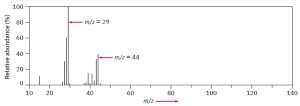
Figure 32.1 Mass Spectrum of Propane
Mass spectral fragmentation patterns are usually complex, and the molecular ion is often not the base peak. The mass spectrum of propane, for instance, shows a molecular ion at m/z = 44 that is only about 30% as high as the base peak at m/z = 29. In addition, many other fragment ions are present.

