4 10.3 Environmental Impacts
Burning petroleum oil products releases emissions such as carbon monoxide (CO), sulfur dioxide (SO2), nitrogen oxides (NOx), and particulate material all of which are air pollutants that impact the environment and human health (see more on air pollution in Chapter 6). Petroleum also emits carbon dioxide which is a greenhouse gas. Exploring and drilling for oil may disturb land and ocean habitats. On land, extensive infrastructure such as road networks, transport pipelines and housing for workers are needed to support a full-scale drilling operation. These can pollute soil and water, fragment habitats, and disturb wildlife [6].
Human-Induced Climate Change
Fossil fuels are made up mainly of hydrogen and carbon. When burned, the carbon combines with oxygen to create carbon dioxide (CO2). The amount of CO2 produced depends on the carbon content of the fuel. For example, for the same amount of energy produced, natural gas produces about half and petroleum produces about three-fourths of the amount of CO2 produced by coal. Energy-related CO2 emissions resulting from the combustion of coal, petroleum, and natural gas account for about 80% of total U.S. human-caused (anthropogenic) greenhouse gas (GHG) emissions. There are many sources of non-energy CO2 emissions, but those emissions account for a relatively small share of total GHG emissions [6].
Burning fossil fuels releases greenhouse gases. With more greenhouse gases trapping heat, average annual global temperatures are rising. Warming temperatures are bringing changes to much of the planet, including Arizona. Precipitation is decreasing, temperatures are rising, the snowpack is changing and the ecology of the state is responding to these changes. Globally, glaciers are retreating, sea level is rising, biomes are moving north and we are seeing more extreme weather events. We will study climate change in more detail in a subsequent chapter.
Air pollution
Fossil fuels are burned in most motor vehicles and power plants. These nonrenewable resources power nearly all manufacturing and other industries. Pure coal and petroleum emit carbon dioxide (CO2) and water. CO2 is a potent greenhouse gas that regulates the climate system. In addition, most of the time, fossil fuels are impure and do not burn completely. The incomplete chemical reactions produce pollutants. The natural impurities, or additives, found in fossil fuels are another source of pollutants released to the environment when burning. These pollutants include carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), and hydrocarbons.
- Carbon oxides include carbon monoxide (CO) and carbon dioxide (CO2). Both are colorless, odorless gases. CO is toxic to both plants and animals. CO and CO2 are both greenhouse gases.
- Nitrogen oxides (NOX) are produced when nitrogen and oxygen from the atmosphere react at high temperatures. This occurs in hot exhaust gas from vehicles, power plants, or factories. Nitrogen oxide (NO) and nitrogen dioxide (NO2) are greenhouse gases. Nitrogen oxides contribute to acid rain.
- Sulfur oxides include sulfur dioxide (SO2) and sulfur trioxide (SO3). These form when sulfur from burning coal reaches the air and reacts with oxygen. Sulfur oxides are components of acid rain.
- Impurities and additives are released upon fossil fuel burning. For example, lead (Pb) was once widely used in automobile fuels, paint, and pipes. This heavy metal causes brain damage or blood poisoning. Fortunately, lead is no longer added to gasoline. Mercury is another impurity commonly found in coal. Similar to lead, its neurotoxicity and genetic damage can harm humans for generations. Mercury is emitted as a gas, but it becomes a droplet as it cools, eventually falling to the ground. If they fall into sediments, bacteria convert them to the most dangerous form of mercury: Methyl mercury. This is a highly toxic form of mercury.
- Volatile organic compounds (VOCs) are mostly hydrocarbons. Important VOCs include methane (a naturally occurring greenhouse gas that is increasing because of human activities), chlorofluorocarbons (human-made compounds that are being phased out because of their effect on the ozone layer), and dioxin (a byproduct of chemical production that serves no useful purpose but is harmful to humans and other organisms).
- Particulates are solid particles having small diameter sizes measured in micrometers or microns (µm). An indicator of air quality is the PM index. You may have seen or heard about PM10 or PM2.5. PM stands for particle matter and the number in the subscript indicates the particle size in microns. Particles measuring between 10-2.5 µm are considered “coarse” while particles with diameters less than 2.5 are considered “fine”. Notice that we are not talking about composition, rather we are differentiating particles based on their size. This useful distinction allows to predict their behavior in the environment and their impacts on organisms. Examples of particulate matter include ash, dust, and even fecal matter. They are commonly formed from the combustion of fossil fuels and can produce smog. . Particulates can contribute to asthma, heart disease, and some types of cancers.To learn more visit the EPA ‘What is PM?’ site
Photochemical smog

A type of air pollution produced by the chemical reaction between some molecules in auto-exhaust or oil refinery emissions, and sunshine. Photochemical smog consists of more than 100 compounds, most importantly ozone.
Air pollution could have been problematic when ancient people burned wood for heat and cooking fires in enclosed spaces such as caves and small tents or houses. But the problem became widespread when the steam engine was invented and fossil fuels such as coal burned massively during the Industrial Revolution. An air pollution crisis exploded in the developed nations in the mid-20th century. Coal smoke and auto exhaust combined to create toxic smog that in some places caused lung damage and sometimes death. In Donora, Pennsylvania, in October 1948, 20 people died and 4,000 became ill when coal smoke was trapped by an inversion. In London in December 1952, the “Big Smog” killed 4,000 people over five days. Many thousands more likely died of health complications from the event in the next several months.
The Clean Air Act
The terrible events in Pennsylvania and London, plus the recognition of the hazards of photochemical smog, led to the passage of the Clean Air Act in 1970 in the United States. The act now regulates 189 pollutants. The six most important pollutants regulated by the Act are ozone, particulate matter, sulfur dioxide, nitrogen dioxide, carbon monoxide, and the heavy metal lead. Other important regulated pollutants include benzene, perchloroethylene, methylene chloride, dioxin, asbestos, toluene, and metals such as cadmium, mercury, chromium, and lead compounds.
What is the result of the Clean Air Act? In short, the air in the United States is much cleaner. Visibility is better and people are no longer incapacitated by industrial smog. However, despite the Act, industry, power plants, and vehicles put 160 million tons of pollutants into the air each year. Some of this smog is invisible and some contribute to the orange or blue haze that affects many cities.
Ocean acidification
The ocean absorbs roughly half of all carbon dioxide added to the atmosphere. By means of chemical reactions with water, the CO2 (gas) ends up as bicarbonate in the water (HCO3). The reaction frees up a hydrogen atom, which acts as an acid, and lowers the pH of water (Fig. 10.10).
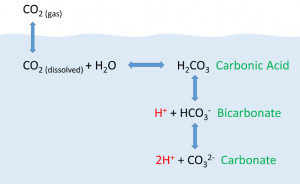
As anthropogenic sources of atmospheric CO2 have increased since the Industrial Revolution, the oceans have been absorbing an increasing amount of CO2, and researchers have documented a decline in ocean pH from about 8.2 to 8.1 in the last century. This may not appear to be much of a change, but pH is on a logarithmic scale, and this decline represents a 30% increase in acidity. Even at a pH of 8.1 the ocean is not actually acidic; the term “acidification” refers to the fact that the pH is becoming lower, i.e. the water is moving towards more acidic conditions [7].
Why is this important? Declining pH can impact many biological systems. Of particular concern are organisms that secrete calcium carbonate shells or skeletons, such as corals, shellfish, and may planktonic organisms. At lower pH levels, calcium carbonate dissolves, eroding the shells and skeletons of these organisms [7]. Another consequence is the reduction of free carbonate ions in the water. Since these are key building blocks for organisms, its availability limits the organisms capacity to grow.

Oil leaks and spills
The deepwater Horizon spill, April 10, 2010, was the largest marine oil spill in history. Listen to Prof. Donald Boesch in the video. He explains why offshore drilling is not safer a decade later.
Human-caused oil spills in rivers and oceans harm ecosystems. Natural oil seepages do occur and may be a significant source of oil that enters the environment globally, but they are slow, small, and spread out over large areas, and the ecosystem has adapted to them. Spills from tankers or well spills have more catastrophic impacts. The quantity of oil spilled during accidents has ranged from a few hundred tons to several hundred thousand tons but even small spills have been shown to have a great impact on ecosystems [6].
Oil spills at sea are generally much more damaging than those on land, since they can spread for hundreds of nautical miles in a thin oil slick which can cover beaches with a thin coating of oil. This can kill sea birds, mammals, shellfish and other organisms it coats. Oil spills on land are more readily containable if a makeshift earth dam can be rapidly bulldozed around the spill site before most of the oil escapes, and land animals can avoid the oil more easily. The 13 amount of oil spilled from ships dropped significantly during the 1990s partly because new ships were required to have a double-hull lining to protect against spills [6].
Leaks also happen when we use petroleum products on land. For example, gasoline sometimes drips onto the ground when people are filling their gas tanks, when motor oil gets thrown away after an oil change, or when fuel escapes from a leaky storage tank. When it rains, the spilled products get washed into the gutter and eventually flow to rivers and into the ocean. Another way that oil sometimes gets into water is when fuel is leaked from motorboats and jet skis. When a leak in a storage tank or pipeline occurs, petroleum products can also get into the ground, and the ground must be cleaned up. To prevent leaks from underground storage tanks, all buried tanks are supposed to be replaced by tanks with a double lining [6].
Plastic Pollution
Oil is primarily used as a fuel for transportation. Oil is also used to manufacture plastics and other synthetic compounds ubiquitous to our everyday life [6]. Unfortunately, plastics persist in the environment and are rapidly accumulating everywhere, reaching the deepest parts of the ocean and entering all the biosfere (including our bodies). Plastic materials do not degrade. They can be broken down into smaller pieces that we cannot see but that are reaching all corners of our globe.
https://youtube.com/watch?v=WoRrqa1nxi4
resulting from human activities

