10.2 Fossil Fuels
Carolina Londono Michel
Hydrocarbons
Fossil fuels are extractable sources of stored energy created by ancient ecosystems. The natural resources that typically fall under this category are coal, oil (petroleum), and natural gas. Living organisms such as plants, phytoplankton, algae, and cyanobacteria captured sunlight energy via photosynthesis. We could refer to these resources as fossil solar energy since the energy of the sun in the past has been converted into chemical energy within a fossil fuel. Fossil fuels are molecular chains of hydrogen and carbon, thus the name Hydrocarbons. Of course, as the energy is used, the once stored carbon enters the atmosphere, causing consequences for our climate system. Fossil fuels account for much of the energy used in the world [1].
Transforming matter from living organisms into hydrocarbons (fossil fuels) is complex. You may have heard that petroleum formed from dinosaur bones, but that is inaccurate. Most oil formed during the dinosaur era, the Mesozoic Era, but T-Rex and the megafauna contributed little to it. The real energy heroes are the plants and marine algae that lived at that time. But why? How?
During much of the Mesozoic, the earth’s climate was tropical, warm, and without polar ice caps. It was Plankton’s paradise, with lots of room to live and plenty of sunshine to live by. The continents were in different positions, maximizing the ocean surface where the algae lived and changing the way the ocean currents flowed. In fact, there was not much movement at the bottom of the ocean during the Mesozoic! (Fig 10.3). Without mixing and vigorous circulation, the oxygen did not reach the ocean floor and vast areas of it were anoxic. These conditions facilitated the chemical reactions needed to form hydrocarbons. The low oxygen conditions also prevail in swamps, bogs, marshes which were common during this Paleozoic (the era before the Mesozoic). In fact, most of our coal resources were deposited during the geologic period Carboniferous (part of the Paleozoic era); Carboniferous means coal-bearing, indicating the widespread deposition and formation of coal.

Cooking fossil organic matter in Earth’s kitchen
How does Earth turn organic matter into hydrocarbons? First, plants and plankton die and fall to the bottom of the swamp or sea, respectively, where piles of sediments quickly bury them. They do not decompose. Decomposition needs oxygen and because their environments lack oxygen, the organic matter is not oxi
dized (decomposed) but reduced. (Rapid burial is the key). The chemical energy within the organisms’ tissues is transferred to surrounding geologic material (sediments, minerals). Again, the key here is that organisms DO NOT decompose, instead they transform into something else under the right heat and pressure conditions. It actually takes a defined range of temperature and pressure conditions over defined amounts of time to produce hydrocarbons, “the oil generation window“! Heat and pressure applied after the burial of sediments also can transform the organic matter trapped in them into “higher quality” materials (brown coal to anthracite, oil to gas) and/or migration of mobile materials, such as gas.
Conventional Fossil Fuels: Oil and Gas
Organic-rich sediments deposited in shallow marine environments formed petroleum and gas, think shallow seas close to the continents. As the organic-rich sediments are buried, the sediments become lithified, that is, converted into rock (typically shale, mudstone, or limestone) while the organic matter transforms into kerogen. The kerogen then changes into oil. The shale, mudstone, or limestone in which hydrocarbons form are the source rock. Once created, the oil and gas leak out of the source rock and migrate to a different rock, usually located above the source (Fig 10.5). The rock that hosts the hydrocarbons is called the reservoir rock.
Reservoir rocks are typically relatively permeable allowing fluids to enter and move; this also facilitates recovery of the oil or gas. Sometimes, the oil liquids and gases make it all the way to the surface, where they sip. Ancient people used oil from sips in different ways. The oil and gas are oxidized at the surface and the carbon eventually returns to the atmosphere. In other cases, the fluids and gasses do not make it to the surface, they are trapped underground. An impermeable layer (e.g. mudstone or claystone) can seal the reservoir, impeding flow and storing the hydrocarbons underneath the Earth. The liquids and gases that are trapped within reservoirs become layered based on their density, with gas rising to the top, oil below it, and water underneath. [3]
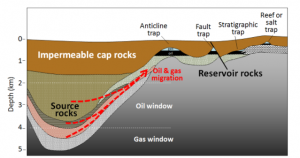
A trap is a combination of a subsurface geologic structure and an impervious layer that helps block the movement of oil and gas and concentrates it for later human extraction. The development of a trap could be a result of many different geologic situations. Common examples include: an anticline or dome structure, an impermeable salt dome, or a fault-bounded stratigraphic block (porous rock next to non-porous rock). The different traps have one thing in common: they pool the fluid fossil fuels into a configuration in which extraction is more likely to be profitable. Oil or gas in strata outside of a trap renders extraction is less viable.
Coal

Coal has been used by humans for at least 6000 years, mainly as a fuel source. Coal resources in Wales are often cited as a primary reason for the rise of Britain (and later, the United States) in the Industrial Revolution [1]. Coal, the first fossil fuel to be widely used, forms mostly on land in swampy areas next to rivers and deltas in areas with humid tropical to temperate climates. The vigorous growth of vegetation leads to an abundance of organic matter that accumulates within stagnant water and thus does not decay and oxidize.
This situation, where the dead organic matter is submerged in oxygen-poor water, must be maintained for centuries to millennia in order for enough material to accumulate to form a thick layer. At some point, the swamp deposit is covered with more sediment — typically because a river changes its course or sea level rises. As more sediments are added, the organic matter starts to become compressed and heated.
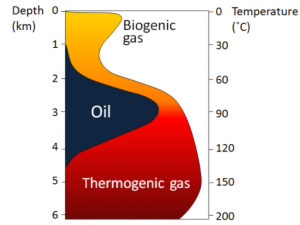
Low-grade lignite coal forms at depths between a few 100 m and 1,500 m and temperatures up to about 50°C. At between 1,000 m to 5,000 m depth and temperatures up to 150°C m, bituminous coal forms (Figure 20.3.1d). At depths beyond 5,000 m and temperatures over 150°C, anthracite coal forms.
Most coal resources in Arizona are in the Navajo reservation, known as the Kayenta mine (See case study). According to the US Energy Information Administration, the production of coal in the US has decreased due to cheaper prices of competing energy sources and recognition of its negative environmental impacts, including increased very fine-grained particulate matter, greenhouse gases, acid rain, and heavy metal pollution. Seen from this point of view, the coal industry is unlikely to revive [1].
Backyard Geology: Coal in Navajo Nation
The Navajo Generating Station (NGS) opened in 1974 to power the Southwest and to send south water from the Colorado River (toward Phoenix and Tucson). The 2,250 Megawatts of electricity powered big cities, like Los Angeles, Phoenix, and Las Vegas.
The Kayenta coal Mine, in Black Mesa, AZ supplied the coal to power the Navajo Generating Station (NGS); Peabody Energy, a multinational company, operated it. The Black Mesa mine also supplied coal to the Mohave Generating Station, in Nevada. They moved the coal in the form of a slurry, using nearly 3.3 million gallons of water pumped from the Navajo Aquifer. The mining operation dried springs on which the Navajo relied for thousands of years. To cool the plant and to control dust pollution, the NGS used 32,000 acre-feet of Colorado River water per year. This same amount of water would supply ~64,000 homes. Water is scarce in the desert, and the Navajo aquifer was the only source of drinking water for the population. Now the Navajo must haul water for their livestock, and for them. Yet, some Diné (Navajo) could find jobs at home and built their lives around the mining activity. This was an incentive and an advantage for them.

Then the coal industry lost its ground due to air pollution concerns and the availability of new unconventional resources that made their price decline. The price of gas coal dipped, they were no longer profitable. Since 2010, more than half of coal-fired electricity plants have closed or will soon be closed. Coal is not coming back.
In 2017, the NGS plant’s operator (SRP) announced that they would shut off the plant by December 2019. This would mean closing the Kayenta mine as well. The announcement stirred the Navajo Nation. At least 700 Native people worked on the coal plant and coal mine, and over 2000 had jobs linked to the coal industry. For many of them, the closing meant losing their way of living. Further, the cuts in the revenue received by the Navajo government would leave a big fiscal hole for the Tribal Nation. The Navajo economy had become dependent on coal some tried to stop the closing.
But not everyone agreed. Opponents to the mine received the news well. The coal mine scarred the landscape, desecrated Black Mesa, polluted the air, left them without water, and caused an increased rate of diseases such as cancer, heart failure, and asthma; the Clean Air Act linked the disease to the coal operations. What’s most, the Navajo were direct beneficiaries of the energy their resources and land generated. Despite owning a generation plant, one-third of their homes lack power and water access.
In 2020 the NGS plant was demolished, marking the decline of coal in the west. But they left the Navajo Nation to pay for all the environmental impacts. Peabody has requested the Office of Surface Mining to delay much of the reclamation work, until 2022. The company could have maintained jobs by employing them to return the open coal pits to workable land. But they didn’t, instead, they ask for an extension. Recall our Geoethics topic, on Ch. 1. Do you think Peabody behaved in an ethical way?
When raw goods on reservation lands are purchased by powerful actors from underserved communities under unfavorable conditions at a fraction of their eventual wealth, this is called resource colonialism (Curley, 2017). And unfortunately, the NGS and the coal mines associated with it are an example of this type of costly extraction.
Sources:
Curley, A. (June 28, 2017) The Navajo Nation’s coal economy was built to be exploded. High Country News. https://www.hcn.org/articles/analysis-tribal-affairs-cleaning-up-coal-on-navajo-nation
Kutz, J. (Feb. 1, 2021). The fight for an equitable energy economy for the Navajo Nation. High Country News. https://www.hcn.org/issues/53.2/south-coal-the-fight-for-an-equitable-energy-economy-for-the-navajo-nation
Rainey, J. (2017). Lighting the West, dividing a tribe. NBC news. https://www.nbcnews.com/specials/navajo-coal/
Listen to Anita Yazzie as she recalls the water, plant and environmental services that were lost due to the coal operation.
Unconventional Fossil Fuels
Conventional oil and gas (pumped from a reservoir) are not the only way to obtain hydrocarbons. The next few sections are known as unconventional petroleum sources, though, they are becoming more important as conventional sources increase in scarcity.
Tar sands or Oil Sands

Tar sands, or sandstones that contain viscous (like tar) materials, cannot be drilled and pumped out of the ground, unlike conventional oil changes that have taken place at the surface [3]. Tar sands contain the fossil fuel bitumen, which can be pumped as a fluid only at very low rates of recovery and only when heated or mixed with solvents. Thus injections of steam and solvents, or direct mining of the tar sands for later processing can be used to extract the tar from the sands. Alberta, Canada is known to have the largest reserves of tar sands in the world. Note: an energy resource becomes uneconomic once the total cost of extracting it exceeds the revenue which is obtained from the sale of extracted material [1].
Oil sands are very controversial from an environmental and social perspective. The environmental cost of their extraction is very high. Since the oil is so viscous, it requires heat to make it sufficiently liquid to process. This energy comes from gas; approximately 25 m3 of gas is used to produce 0.16 m3 (one barrel) of oil. The other environmental cost of oil sands production is the devastation of vast areas of land where strip-mining (open pit) is taking place and tailings ponds are constructed, and the unavoidable release of contaminants into the groundwater and rivers of the region.
Oil Shale
Fracking
Another process that is used to extract the oil and gas from shale and other unconventional tight resources is called hydraulic fracturing, better known as fracking. In this method, high-pressure injections of water, sand grains, and added chemicals are pumped underground, creating and holding open a fracture, or break within a rock that has no relative movement between the sides.
Caused by cooling, pressure release, tectonic forces, etc., fractures in the rocks, aid in the release of the hard-to-access fluids, mostly natural gas. This is more useful in tighter sediments, especially shale, which has a high porosity to store the hydrocarbons but low permeability to transmit the hydrocarbons. Fracking has become controversial due to the potential for groundwater contamination and induced seismicity, and represents a balance between public concerns and energy value [1].
Energy development often requires substantial amounts of water, and hydraulic fracturing is no exception. Water is needed not only for the traditional drilling process but also for the actual fracturing as well. Water is first mixed with chemicals and fine sands then pumped at extremely high pressure into the shale rock to fracture it, forming pathways for the oil and gas to reach the well. The water is then recovered, along with the oil and gas.
There are concerns regarding the potential contamination of fresh groundwater resources from oil and gas extraction wells that use hydraulic fracturing; either from the petroleum resource being produced or from the chemicals introduced in the fracturing process. Fracking fluid flowback – the fluid pumped out of the well and separated from oil and gas – not only contains the chemical additives used in the drilling process but also contains heavy metals, radioactive materials, volatile organic compounds (VOCs) and hazardous air pollutants such as 12 benzene, toluene, ethylbenzene and xylene. In some cases, this contaminated water is sent to water treatment plants that are not equipped to deal with some of these classes of contamination [6].
An abbreviated History of Fossil Fuels
Fuel formed from biomass by a
process taking millions of years or longer.
A major subdivision of geologic time from 251 Ma to 65 Ma; characterized by dinosaurs.
Without oxygen
The solidification of loose sediment materials as solid sedimentary rock through compacting pressures and cementation.
A clastic sedimentary rock made of very fine-grained sediments such as muds, clays, and silts.
A rock composed of consolidated mud (clay, silt, or a combination of the two.)
An organic or chemical sedimentary rock that is primarily composed of calcium carbonate (CaCO3). Limestone is a subgroup of rocks that includes chalk, coquina, and fossiliferous limestone.
fossilized insoluble organic material that can be converted to petroleum.
Having permeability. The relative ease of fluid flow in a porous material
An organic sedimentary rock formed by the compaction of plant and animal organic matter over millions of years.
Low-grade type of coal with less than 70% carbon
a medium-grade type of coal with 70 to 92% carbon
a high grade of coal (92 to 98% carbon) that is formed from deep burial and weak metamorphism
Interventions to make the landscape useful again
Sands or sandstones that contain high-viscosity petroleum
A fluid (such as lava or magma) that is highly resistant to flow or movement due mostly to composition.
having numerous interstices, whether connected or isolated
rocks that cement together from weathering products, either from sediments or chemical ions in water.

