9.3 Arizona: The Copper State
Carolina Londono Michel

Arizona’s economy thrives on mining. Arizona’s largest copper deposits rank among the biggest in the world, making the state one of the top copper suppliers for the last 150 years. In 2020, Arizona ranked second for the value of non-fuel mineral production in the United States, it produced more than $7 billion worth of non-fuel mineral commodities (USGS, 2021). The principal non-fuel mineral commodities are cement (portland), copper, molybdenum, concentrates, sand and gravel (construction), stone (crushed). (USGS, 2021) Arizona also produces gemstones, it is world-famous for its turquoise, peridotite, and perlite.
A metal deposit is a body of rock in which one or more metals have been concentrated to the point of being economically viable for recovery [3]. Some background levels of important metals in average rocks are shown in Table 1, along with the typical grades, or concentrations, necessary to make a viable deposit, and the corresponding concentration factors. For example, if we look at copper we can see that while average rock has around 40 ppm (parts per million) of copper, a grade of around 10,000 ppm or 1% is necessary to make a viable copper deposit. In other words, copper ore has about 250 times as much copper as typical rock. For the other elements in the list, the concentration factors are much higher. For gold, it’s 2,000 times and for silver, it’s around 10,000 times.
It is clear that some very significant concentration must take place to form a mineable deposit. This concentration may occur during the formation of the host rock, or after the rock forms, through a number of geologic processes. There is a very wide variety of ore-forming processes, and there are hundreds of types of mineral deposits. The origins of a few of them are described below.
| Metal | Typical Background Level | Typical Economic Grade* | Concentration Factor |
| Copper | 40 ppm | 10,000 ppm (1%) | 250 times |
| Gold | 0.003 ppm | 6 ppm (0.006%) | 2,000 times |
| Lead | 10 ppm | 50,000 ppm (5% | 5,000 times |
| Molybdenum | 1 ppm | 1,000 ppm (0.1%) | 1,000 times |
| Nickel | 25 ppm | 20,000 ppm (2%) | 800 times |
| Silver | 0.1 ppm | 1,000 ppm (0.1%) | 10,000 times |
| Uranium | 2 ppm | 10,000 ppm (1%) | 5,000 times |
| Zinc | 50 ppm | 50,000 ppm (5%) | 1,000 times |
| It is important to note that the economic viability of any deposit depends on a wide range of factors including its grade, size, shape, depth below the surface, and proximity to infrastructure, the current price of the metal, the labor and environmental regulations in the area, and many other factors. Source: [3]
|
|||
Native Americans Contributions to Mining
Native Americans accrued and passed empirical knowledge by direct interaction with the natural world. This body of

knowledge is variously referred to as Native science, ethnoscience, and Traditional Ecological Knowledge, TEK. TEK is practical, sophisticated, and can share the purposes and benefits of mainstream geology and ecology.
Indigenous peoples have expertise with diverse minerals. They hold specialized knowledge about mineral properties, qualities, and their occurrences on the land. For the Diné, a Tribal Nation of the Southwest, turquoise has cultural, religious, social, and economic relevance. The Diné (Navajo) word for turquoise is dotl’ izhi. The Navajo have long known where to find turquoise, how to extract it, and how to work with it to produce a myriad of objects. Navajo jewelry is famous around the globe for its beauty and high quality.
Quarrying, surface mining, and metallurgical knowledge have been present throughout the Americas for millennia. Native people applied TEK to manage and use lands and technologies that make their lives better (Cajete,2010). Further, Native miners and metalworkers improved imported European technology. They invented ore-crushing and automated coin-minting machines previously unknown in Europe. The metallurgical knowledge of South American tribes raised the efficiency of silver extraction and was apt for high altitudes, where it originated. These contributions to mining technology boosted the development of the modern industrial era (Cajete, 2010).
Metalogenesis. The Origin of Metal Deposits.
Volcanogenic Massive Sulfides VMS
Certain volcanic processes concentrate minerals and the resulting deposits are called volcanogenic deposits. A particular kind of these mineral deposits is dubbed “Volcanogenic Massive Sulfides (VMS)” given the unusually abundant mass of sulfide minerals.
Copper mined in Jerome, central Arizona is mined from VMS deposits associated with ancient submarine volcanoes. Briefly, 1,750 to 1,650 million years. ago, in the Precambrian, the SW part of the United States did not exist. Instead of continental land, Arizona was under the ocean. The oceanic floor was active, with volcanoes and associated underwater hot springs that spewed hot liquids loaded with metals. Beneath the volcanoes and hot springs lied a heat source (magmatic heat source in Fig 9.14). Ocean water can circulate through cracks and fractures within oceanic rocks, that is, there is groundwater flow under the ocean too! The groundwater that penetrates the oceanic rocks travels down, absorbing heat from the magmatic source, the deeper down it travels, the hotter it gets and at some point rises up (see figure 9.14). You may remember a lava lamp and how the heat makes the blobs move when they get hot, they rise, sinking again as they lose heat.
The sulfide minerals (including pyrite (FeS2), sphalerite (ZnS), chalcopyrite (CuFeS2), and galena (PbS)), are generally present in very high concentrations in the oceanic rocks around the volcanic system. The hot groundwater mobilizes the metals out of the host rock, in a process called leaching. Recall that water is a universal solvent, its special properties allow water to carry and move metals from the host rocks to other places, the superheated temperatures (250° to 300°C), increase this behavior. When the hot, metal-loaded groundwater rises, it sips out through springs. But it encounters cold ocean water and the extreme temperature drop causes metallic minerals precipitation around the vents. The minerals that precipitate contain copper, lead, zinc, silver, and gold. The setting is comparable to what is known today as “black smokers”; submarine hot springs that occur near tectonic spreading centers (Ch 2.)
The mineral deposits at Jerome formed in submarine volcanic settings, so you may ask yourself, if that is true then what happened that made those deposits appear on land, in Arizona? The answer is plate tectonics. In short, tectonic forces created what is now the SW part of the US, some of the ancient oceanic floor got pushed into the continents.
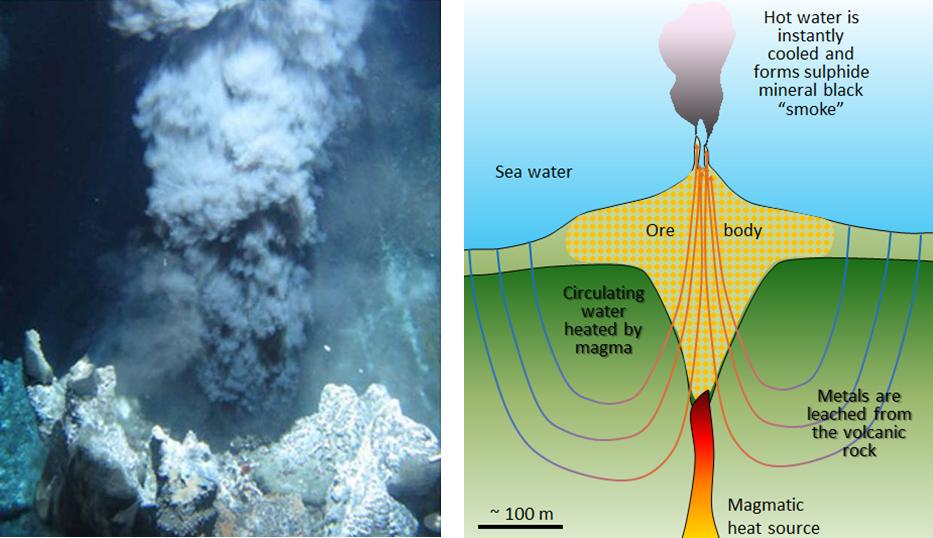
This video shows volcanic hydrothermal vents in the Caribbean. As the super hot fluid mixes with cold water, metal-rich minerals precipitate forming chimney-like structures and concentrating metal deposits. Black smokers are important for a variety of organisms that thrive around them. However, the conditions are so extreme that they are rightfully named ‘extremophiles’ or extreme lovers.
Porphyry Copper Deposits
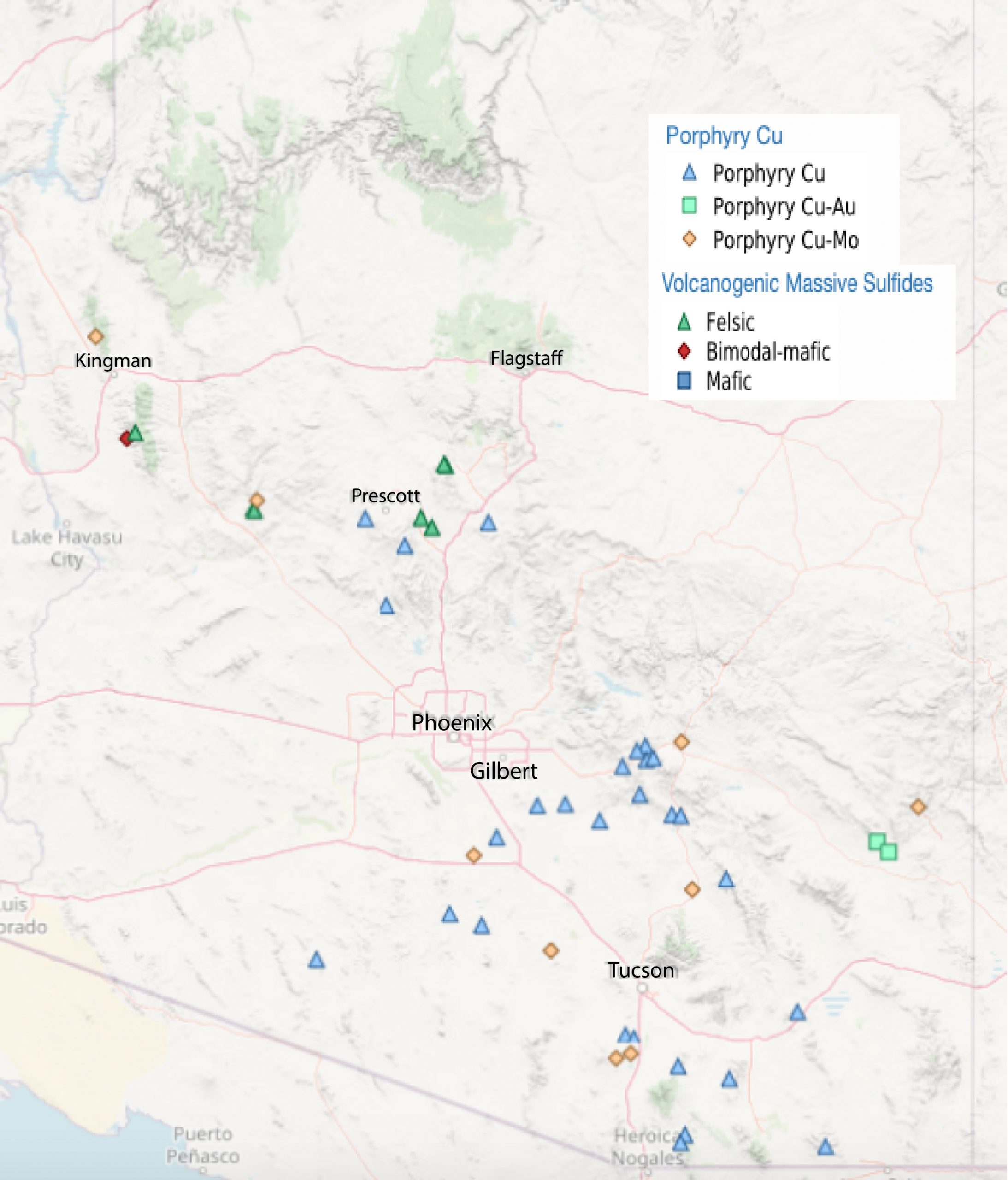
Porphyry deposits are the most important source of copper in British Columbia, the western United States, and Central and South America. Most porphyry deposits also host some gold, and in rare cases, gold is the primary commodity. Arizona examples include several large deposits that occur on a northwest-trending belt (Fig 9.15).
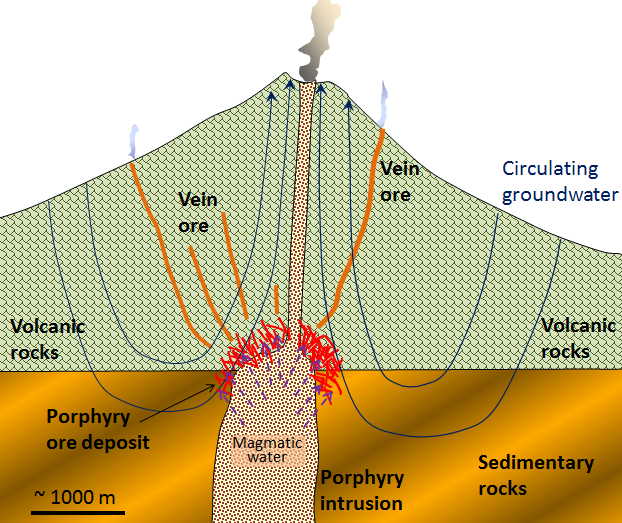
This type of deposit is associated with intrusive igneous rocks (Ch. 4) similar to granite. A porphyry describes a texture in which individual mineral grains about a tenth to a half-inch in size are surrounded by smaller grains that are barely visible to the naked eye. The mineral deposit forms around a cooling felsic stock in the upper part of the crust. Metal enrichment results in part from the convection of groundwater related to the heat of the stock, and also from metal-rich hot water expelled by the cooling magma (Figure 9.15). The host rocks, which commonly include the stock itself and the surrounding country rocks, are normally highly fractured and brecciated. The important ore minerals include chalcopyrite (CuFeS2), bornite (Cu5FeS4), and pyrite in copper porphyry deposits, or molybdenite (MoS2) and pyrite in molybdenum porphyry deposits. Gold is present as minute flakes of native gold [3].
Arizona’s porphyry copper deposits date back to 70 million years ago. Intrusive activity and groundwater concentrated the copper and other metals below the ground. But, our dynamic planet moves things, and thanks to tectonic forces, weathering, and erosion, the rich metal deposits reached the surface millions of years later. Further, the geochemical processes that act during weathering enhance the copper concentration, a process called “secondary enrichment”.
Secondary Enrichment
Secondary enrichment is a process that acts after the first enrichment to further concentrate a metal. Rain precipitation on mineral deposits alters their composition, as you may recall from the weathering and mass wasting chapter. Precipitation can infiltrate and fill in spaces within rocks to become groundwater. In this movement, water can mobilize elements from a host rock and transport them downward, leaching the rock and concentrating elements of interest underground. When the percolating solution reaches the water table, the chemical conditions change and the transported elements precipitate. This leads to an enriched zone below the water table. Copper deposits may have to be enriched in this way to become ore.
the typical level of an element in average rocks or sediments
A mineral compound characterized by the linkage of sulfur with a metal or semi-metal such as galena, PbS, or pyrite, FeS2.
An igneous rock that contains large, visible crystals (phenocrysts) in fine-grained groundmass
originating from a feldspar and silica-rich magma/lava composition.
A relative small intrusive body

