20
Learning Objectives
- Identify the anatomy of the endocrine system
- Describe the main functions of the endocrine system
- Spell the medical terms of the endocrine system and use correct abbreviations
- Identify the medical specialties associated with the endocrine system
- Explore common diseases, disorders, and procedures related to the endocrine system
Endocrine System Word Parts
Click on prefixes, combining forms, and suffixes to reveal a list of word parts to memorize for the Endocrine System.
Introduction to Endocrine System

You may never have thought of it this way, but when you send a text message to two friends to meet you at the dining hall at six, you’re sending digital signals that (you hope) will affect their behaviour—even though they are some distance away. Similarly, certain cells send chemical signals to other cells in the body that influence their behaviour. This long-distance intercellular communication, coordination, and control is critical to maintain equilibrium (homeostasis). This intercellular activity is the fundamental function of the endocrine system.
Watch this video:
Media 20.1 Endocrine System, Part 1 – Glands & Hormones: Crash Course A&P #23 [Online video]. Copyright 2015 by CrashCourse.
Endocrine System Medical Terms
Anatomy (Structures) of the Endocrine System
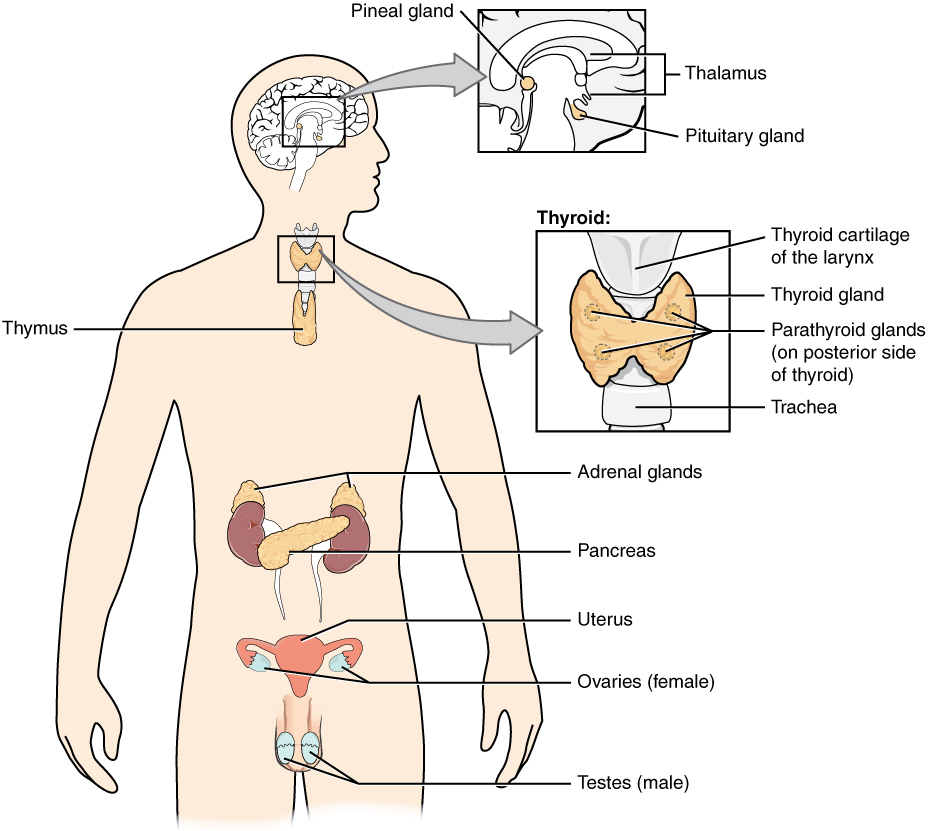
The endocrine system consists of cells, tissues, and organs that secrete hormones as a primary or secondary function. The endocrine gland is the major player in this system. The primary function of the endocrine gland is to secrete hormones directly into the surrounding fluid. The surrounding fluid (interstitial fluid) and the blood vessels then transport the hormones throughout the body. The endocrine system includes the pituitary, thyroid, parathyroid, adrenal, and pineal glands (see Figure 20.2). Some of these glands have both endocrine and non-endocrine functions. For example, the pancreas contains cells that function in digestion as well as cells that secrete the endocrine hormones like insulin and glucagon, which regulate blood glucose levels. The hypothalamus, thymus, heart, kidneys, stomach, small intestine, liver, skin, female ovaries, and male testes are other organs that contain cells with endocrine function. Moreover, fat (adipose) tissue has long been known to produce hormones, and recent research has revealed that even bone tissue has endocrine functions.
The ductless endocrine glands are not to be confused with the body’s exocrine system, whose glands release their secretions through ducts. Examples of exocrine glands include the sebaceous and sweat glands of the skin. As just noted, the pancreas also has an exocrine function: most of its cells secrete pancreatic juice through the pancreatic and accessory ducts to the lumen of the small intestine.
Anatomy Labeling Activity
Physiology (Function) of the Endocrine System
Endocrine Signaling
The endocrine system uses one method of communication called chemical signaling. These chemical signals are sent by the endocrine organs. The endocrine organs secrete chemicals—called hormones—into the fluid outside of the tissue cells (extracellular fluid). Hormones are then transported primarily via the bloodstream throughout the body, where they bind to receptors on target cells, creating a particular response. For example, the hormones released when you are presented with a dangerous or a frightening situation, called the fight-or-flight response, occurs through the release of hormones from the adrenal gland—epinephrine and norepinephrine—within seconds. In contrast, it may take up to 48 hours for target cells to respond to certain reproductive hormones.
In addition, endocrine signaling is typically less specific than neural (nerve) signaling. The same hormone may also play a role in a variety of different physiological processes depending on the target cells involved. For example, the hormone oxytocin generates uterine contractions in women who are in labour. This hormone is also important in generating the milk release reflex during breastfeeding, and may be involved in the sexual response and in feelings of emotional attachment in both males and females.
Generally, the nervous system involves quick responses to rapid changes in the external environment, and the endocrine system is usually slower acting—taking care of the internal environment of the body, maintaining equilibrium (homeostasis), and in controlling reproduction (see Table 20.1). So how does the fight-or-flight response, that was mentioned earlier, happen so quickly if hormones are usually slower acting? It is because the two systems are connected. It is the fast action of the nervous system in response to the danger in the environment that stimulates the adrenal glands to secrete their hormones, epinephrine and norepinephrine. As a result, the nervous system can cause rapid endocrine responses to keep up with sudden changes in both the external and internal environments, when necessary.
| Characteristic | Endocrine System | Nervous System |
|---|---|---|
| Signaling mechanism(s) | Chemical | Chemical/electrical |
| Primary chemical signal | Hormones | Neurotransmitters |
| Distance traveled | Long or short | Always short |
| Response time | Fast or slow | Always fast |
| Environment targeted | Internal | Internal and external |
Other Types of Chemical Signaling
There are four different types of chemical signaling occurring in multicellular organisms: endocrine signaling, autocrine signaling, paracrine signaling, and direct signaling.
In endocrine signaling, hormones secreted into the extracellular fluid spreads into the blood or lymphatic system, and can, therefore, travel great distances throughout the body.
In contrast, autocrine signaling occurs within the same cell. An autocrine (auto- = “self”) is a chemical that triggers a response in the same cell that secreted the chemical. For example, Interleukin-1 (or IL-1), is a chemical signaling molecule that plays a role in inflammation. The cells that release IL-1 also have receptors on their surface that bind IL-1, resulting in autocrine signaling.
Paracrine signaling occurs amongst neighbouring cells. A paracrine (para- = “near”) is a chemical that triggers a response in neighbouring cells. Although paracrines may enter the bloodstream, their concentration is generally too low to elicit a response from distant tissues. A familiar example for those with asthma is histamine, a paracrine that is released by immune cells. Histamine causes the smooth muscle cells of the lungs to constrict, narrowing the airways.
Direct signaling occurs between neighbouring cells across gap junctions. Gap junctions are channels that connect neighbouring cells, that allow small molecules to move between the neighbouring cells.
Concept Check
- Describe the communication methods used by the endocrine system.
- Compare and contrast endocrine and exocrine glands.
- True or false: Neurotransmitters are a special class of paracrines? Explain your answer.
Hormones
Although a given hormone may travel throughout the body in the bloodstream, it will affect the activity only of its target cells; that is, cells with receptors for that particular hormone. Once the hormone binds to the receptor, a chain of events is initiated that leads to the target cell’s response. Hormones play a critical role in the regulation of physiological processes because of the target cell responses they regulate. These responses contribute to human reproduction, growth and development of body tissues, metabolism, fluid, and electrolyte balance, sleep, and many other body functions. The major hormones of the human body and their effects are identified in Table 20.2.
| Endocrine Gland | Associated Hormones | Chemical Class | Effect |
|---|---|---|---|
| Pituitary (anterior) | Growth hormone (GH) | Protein | Promotes growth of body tissues |
| Pituitary (anterior) | Prolactin (PRL) | Peptide | Promotes milk production |
| Pituitary (anterior) | Thyroid-stimulating hormone (TSH) | Glycoprotein | Stimulates thyroid hormone release |
| Pituitary (anterior) | Adrenocorticotropic hormone (ACTH) | Peptide | Stimulates hormone release by adrenal cortex |
| Pituitary (anterior) | Follicle-stimulating hormone (FSH) | Glycoprotein | Stimulates gamete production |
| Pituitary (anterior) | Luteinizing hormone (LH) | Glycoprotein | Stimulates androgen production by gonads |
| Pituitary (posterior) | Antidiuretic hormone (ADH) | Peptide | Stimulates water reabsorption by kidneys |
| Pituitary (posterior) | Oxytocin | Peptide | Stimulates uterine contractions during childbirth |
| Thyroid | Thyroxine (T4), triiodothyronine (T3) | Amine | Stimulate basal metabolic rate |
| Thyroid | Calcitonin | Peptide | Reduces blood Ca2+ levels |
| Parathyroid | Parathyroid hormone (PTH) | Peptide | Increases blood Ca2+ levels |
| Adrenal (cortex) | Aldosterone | Steroid | Increases blood Na+ levels |
| Adrenal (cortex) | Cortisol, corticosterone, cortisone | Steroid | Increase blood glucose levels |
| Adrenal (medulla) | Epinephrine, norepinephrine | Amine | Stimulate fight-or-flight response |
| Pineal | Melatonin | Amine | Regulates sleep cycles |
| Pancreas | Insulin | Protein | Reduces blood glucose levels |
| Pancreas | Glucagon | Protein | Increases blood glucose levels |
| Testes | Testosterone | Steroid | Stimulates development of male secondary sex characteristics and sperm production |
| Ovaries | Estrogens and progesterone | Steroid | Stimulate development of female secondary sex characteristics and prepare the body for childbirth |
Types of Hormones
The hormones of the human body can be divided into two major groups on the basis of their chemical structure. Hormones derived from amino acids include amines, peptides, and proteins. Those derived from lipids include steroids (see Table 20.3). These chemical groups affect a hormone’s distribution, the type of receptors it binds to, and other aspects of its function.
| HORMONE CLASS | COMPONENTS | EXAMPLES |
| Amine Hormone | Amino acids with modified groups (e.g. norepinephrine’s carboxyl group is replaced with a benezene ring) |
 |
| Peptide Hormone | Short chains of linked amino acids |
 |
| Protein Hormone | Long chains of linked amino acides |
 |
| Steroid Hormones | Derived from 4ipid cholesterol |
 |
Amine Hormones
Hormones derived from the modification of amino acids are referred to as amine hormones. Amine hormones are synthesized from the amino acids tryptophan or tyrosine. An example of a hormone derived from tryptophan is melatonin, which is secreted by the pineal gland and helps regulate circadian rhythm.
Peptide and Protein Hormones
Whereas the amine hormones are derived from a single amino acid, peptide and protein hormones consist of multiple amino acids that link to form an amino acid chain. Examples of peptide hormones include antidiuretic hormone (ADH), a pituitary hormone important in fluid balance. Some examples of protein hormones include growth hormone, which is produced by the pituitary gland, and follicle-stimulating hormone (FSH). FSH helps stimulate the maturation of eggs in the ovaries and sperm in the testes.
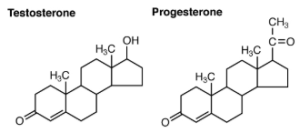
Steroid Hormones
The primary hormones derived from lipids are steroids. Steroid hormones are derived from the lipid cholesterol. For example, the reproductive hormones testosterone and the estrogens—which are produced by the gonads (testes and ovaries)—are steroid hormones. The adrenal glands produce the steroid hormone aldosterone, which is involved in osmoregulation, and cortisol, which plays a role in metabolism.
Like cholesterol, steroid hormones are not soluble in water (they are hydrophobic). Because blood is water-based, lipid-derived hormones must travel to their target cell bound to a transport protein.
Pathways of Hormone Action
The message a hormone sends is received by a hormone receptor, a protein located either inside the cell or within the cell membrane. The receptor will process the message by initiating other signaling events or cellular mechanisms that result in the target cell’s response. Hormone receptors recognize molecules with specific shapes and side groups, and respond only to those hormones that are recognized. The same type of receptor may be located on cells in different body tissues, and trigger somewhat different responses. Thus, the response triggered by a hormone depends not only on the hormone, but also on the target cell.
Once the target cell receives the hormone signal, it can respond in a variety of ways. The response may include the stimulation of protein synthesis, activation or deactivation of enzymes, alteration in the permeability of the cell membrane, altered rates of mitosis and cell growth, and stimulation of the secretion of products. Moreover, a single hormone may be capable of inducing different responses in a given cell.
Factors Affecting Target Cell Response
You will recall that target cells must have receptors specific to a given hormone if that hormone is to trigger a response. But several other factors influence the target cell response. For example, the presence of a significant level of a hormone circulating in the bloodstream can cause its target cells to decrease their number of receptors for that hormone. This process is called downregulation, and it allows cells to become less reactive to the excessive hormone levels. When the level of a hormone is chronically reduced, target cells engage in upregulation to increase their number of receptors. This process allows cells to be more sensitive to the hormone that is present. Cells can also alter the sensitivity of the receptors themselves to various hormones.
Two or more hormones can interact to affect the response of cells in a variety of ways. The three most common types of interaction are as follows:
- The permissive effect, in which the presence of one hormone enables another hormone to act. For example, thyroid hormones have complex permissive relationships with certain reproductive hormones. A dietary deficiency of iodine, a component of thyroid hormones, can therefore affect reproductive system development and functioning.
- The synergistic effect, in which two hormones with similar effects produce an amplified response. In some cases, two hormones are required for an adequate response. For example, two different reproductive hormones—FSH from the pituitary gland and estrogens from the ovaries—are required for the maturation of female ova (egg cells).
- The antagonistic effect, in which two hormones have opposing effects. A familiar example is the effect of two pancreatic hormones, insulin and glucagon. Insulin increases the liver’s storage of glucose as glycogen, decreasing blood glucose, whereas glucagon stimulates the breakdown of glycogen stores, increasing blood glucose.
Concept Check
- Describe how a hormone receptor functions and reacts to messages received.
- Contrast upregulation and downregulation. Are both of these processes necessary? Why or why not?
Regulation of Hormone Secretion
To prevent abnormal hormone levels and a potential disease state, hormone levels must be tightly controlled. The body maintains this control by balancing hormone production and degradation. Feedback loops govern the initiation and maintenance of most hormone secretion in response to various stimuli.
Role of Feedback Loops
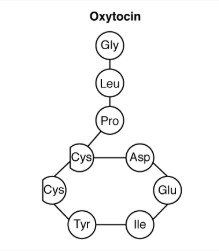
The contribution of feedback loops to homeostasis will only be briefly reviewed here. Positive feedback loops are characterized by the release of additional hormone in response to an original hormone release. The release of oxytocin during childbirth is a positive feedback loop. The initial release of oxytocin begins to signal the uterine muscles to contract, which pushes the fetus toward the cervix, causing it to stretch. This, in turn, signals the pituitary gland to release more oxytocin, causing labor contractions to intensify. The release of oxytocin decreases after the birth of the child.
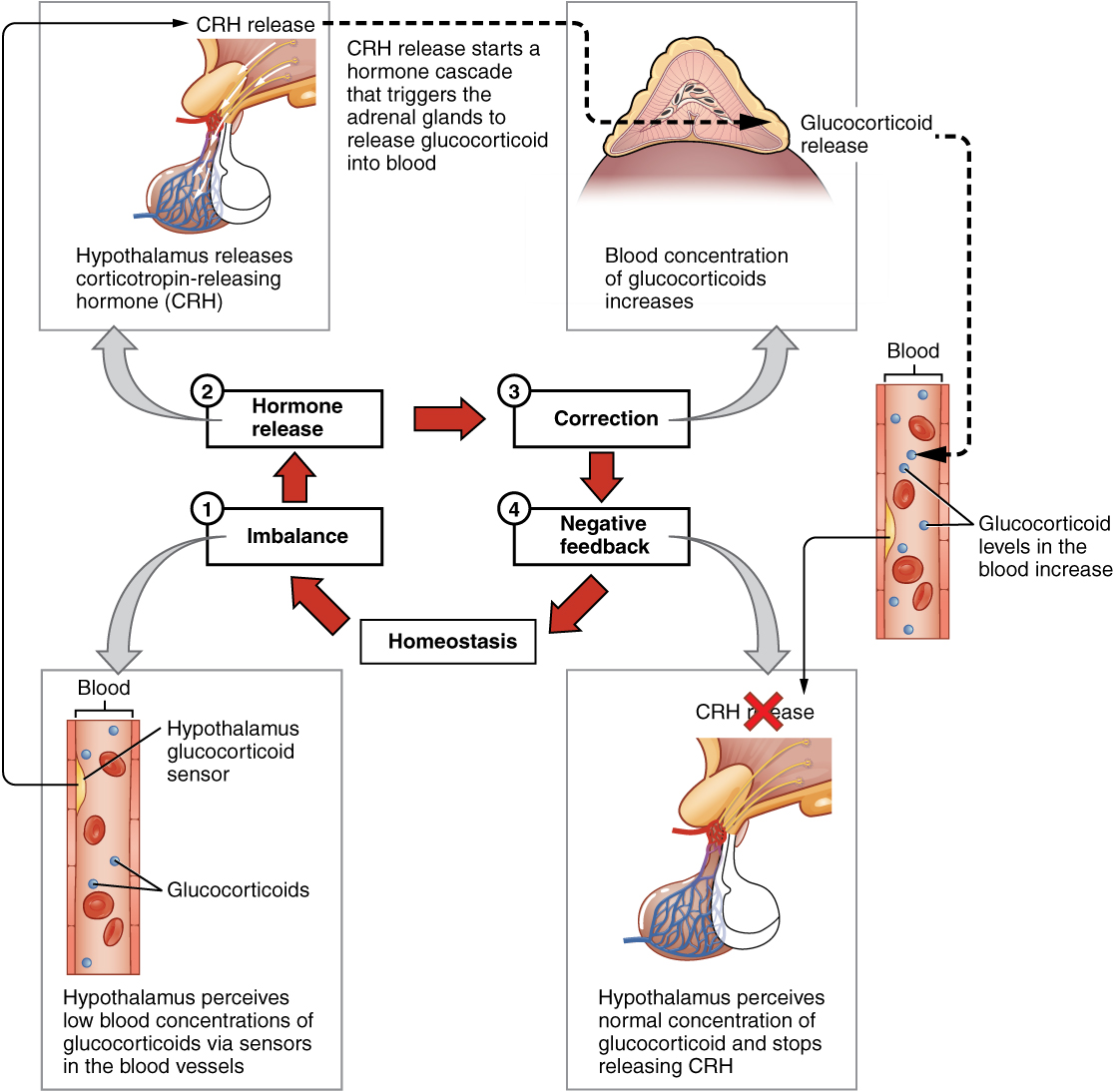
The more common method of hormone regulation is the negative feedback loop. Negative feedback is characterized by the inhibition of further secretion of a hormone in response to adequate levels of that hormone. This allows blood levels of the hormone to be regulated within a narrow range. An example of a negative feedback loop is the release of glucocorticoid hormones from the adrenal glands, as directed by the hypothalamus and pituitary gland. As glucocorticoid concentrations in the blood rise, the hypothalamus and pituitary gland reduce their signaling to the adrenal glands to prevent additional glucocorticoid secretion (see Figure 20.3).
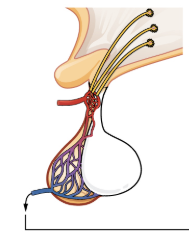
Anterior Pituitary Gland
The anterior pituitary originates from the digestive tract in the embryo and migrates toward the brain during fetal development. There are three regions: the pars distalis is the most anterior, the pars intermedia is adjacent to the posterior pituitary, and the pars tuberalis is a slender “tube” that wraps the infundibulum.
Recall that the posterior pituitary does not synthesize hormones, but merely stores them. In contrast, the anterior pituitary does manufacture hormones. However, the secretion of hormones from the anterior pituitary is regulated by two classes of hormones. These hormones—secreted by the hypothalamus—are the releasing hormones that stimulate the secretion of hormones from the anterior pituitary and the inhibiting hormones that inhibit secretion.
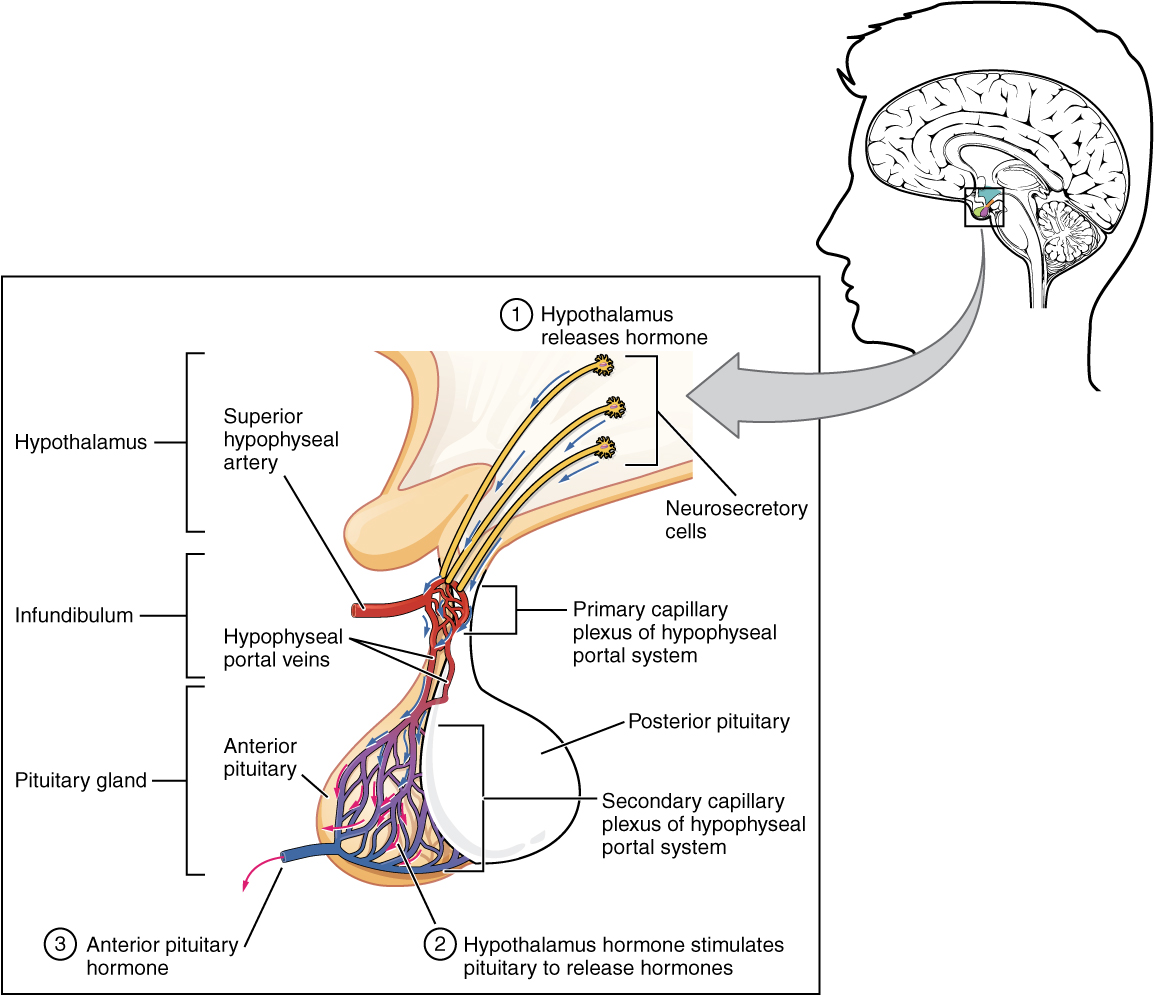
Hypothalamic hormones are secreted by neurons, but enter the anterior pituitary through blood vessels. Within the infundibulum is a bridge of capillaries that connects the hypothalamus to the anterior pituitary. This network, called the hypophyseal portal system, allows hypothalamic hormones to be transported to the anterior pituitary without first entering the systemic circulation. The system originates from the superior hypophyseal artery, which branches off the carotid arteries and transports blood to the hypothalamus. The branches of the superior hypophyseal artery form the hypophyseal portal system (see Figure 20.4). Hypothalamic releasing and inhibiting hormones travel through a primary capillary plexus to the portal veins, which carry them into the anterior pituitary. Hormones produced by the anterior pituitary (in response to releasing hormones) enter a secondary capillary plexus, and from there drain into the circulation.
The anterior pituitary produces seven hormones. These are the growth hormone (GH), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), beta endorphin, and prolactin. Of the hormones of the anterior pituitary, TSH, ACTH, FSH, and LH are collectively referred to as tropic hormones (trope- = “turning”) because they turn on or off the function of other endocrine glands.
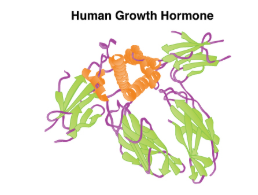
Growth Hormone
The endocrine system regulates the growth of the human body, protein synthesis, and cellular replication. A major hormone involved in this process is growth hormone (GH), also called somatotropin—a protein hormone produced and secreted by the anterior pituitary gland. Its primary function is anabolic; it promotes protein synthesis and tissue building through direct and indirect mechanisms (see Figure 20.5). GH levels are controlled by the release of GHRH and GHIH (also known as somatostatin) from the hypothalamus.
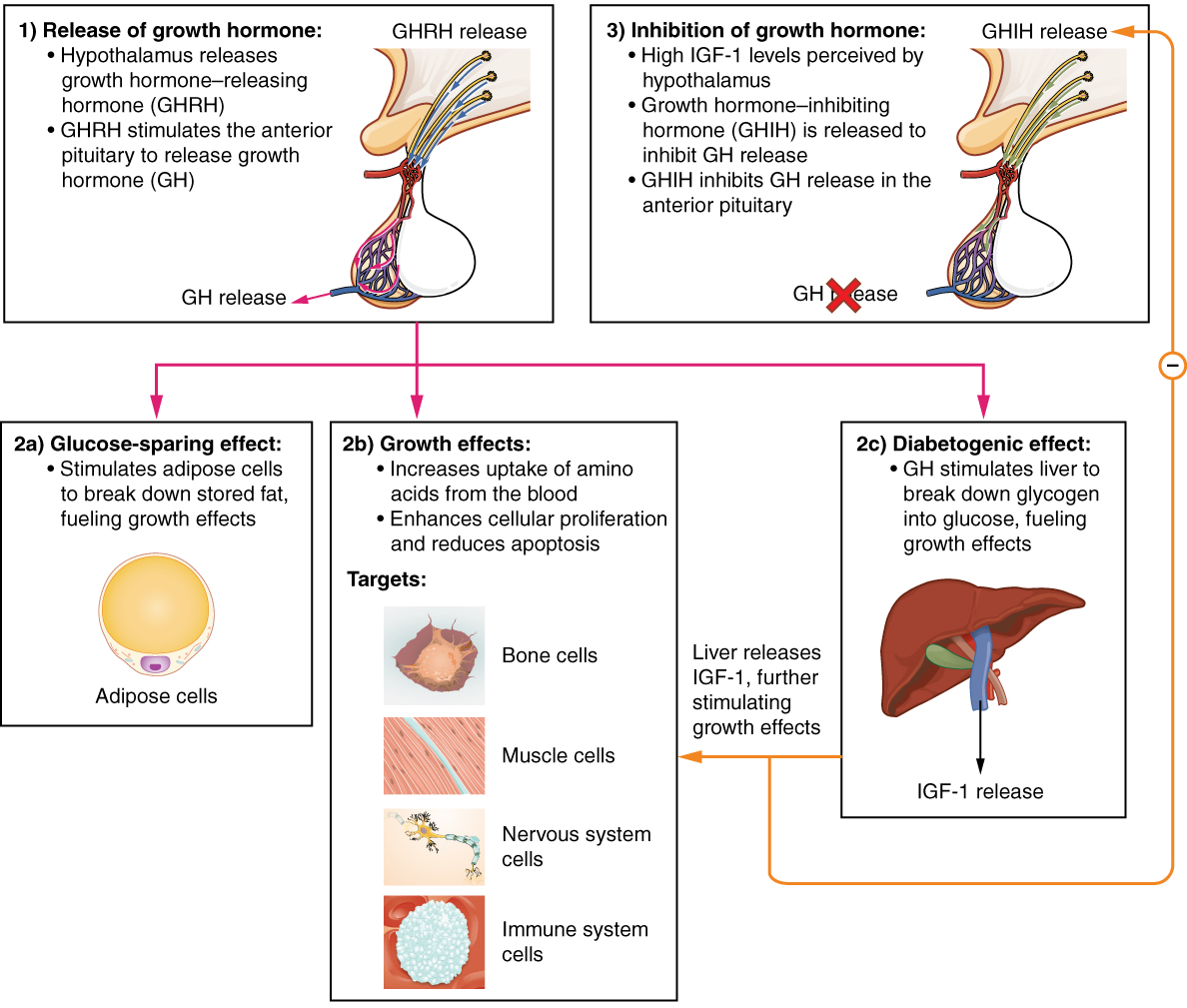
A glucose-sparing effect occurs when GH stimulates lipolysis, or the breakdown of adipose tissue, releasing fatty acids into the blood. As a result, many tissues switch from glucose to fatty acids as their main energy source, which means that less glucose is taken up from the bloodstream.
GH also initiates the diabetogenic effect in which GH stimulates the liver to break down glycogen to glucose, which is then deposited into the blood. The name “diabetogenic” is derived from the similarity in elevated blood glucose levels observed between individuals with untreated diabetes mellitus and individuals experiencing GH excess. Blood glucose levels rise as the result of a combination of glucose-sparing and diabetogenic effects.
GH indirectly mediates growth and protein synthesis by triggering the liver and other tissues to produce a group of proteins called insulin-like growth factors (IGFs). These proteins enhance cellular proliferation and inhibit apoptosis, or programmed cell death. IGFs stimulate cells to increase their uptake of amino acids from the blood for protein synthesis. Skeletal muscle and cartilage cells are particularly sensitive to stimulation from IGFs.
Dysfunction of the endocrine system’s control of growth can result in several disorders. For example, gigantism is a disorder in children that is caused by the secretion of abnormally large amounts of GH, resulting in excessive growth. A similar condition in adults is acromegaly, a disorder that results in the growth of bones in the face, hands, and feet in response to excessive levels of GH in individuals who have stopped growing. Abnormally low levels of GH in children can cause growth impairment—a disorder called pituitary dwarfism (also known as growth hormone deficiency).
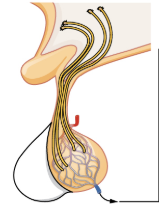
Posterior Pituitary Gland
The posterior pituitary is actually an extension of the neurons of the nuclei of the hypothalamus. The cell bodies of these regions rest in the hypothalamus, but their axons descend as the hypothalamic–hypophyseal tract within the infundibulum, and end in axon terminals that comprise the posterior pituitary (see Figure 20.6).
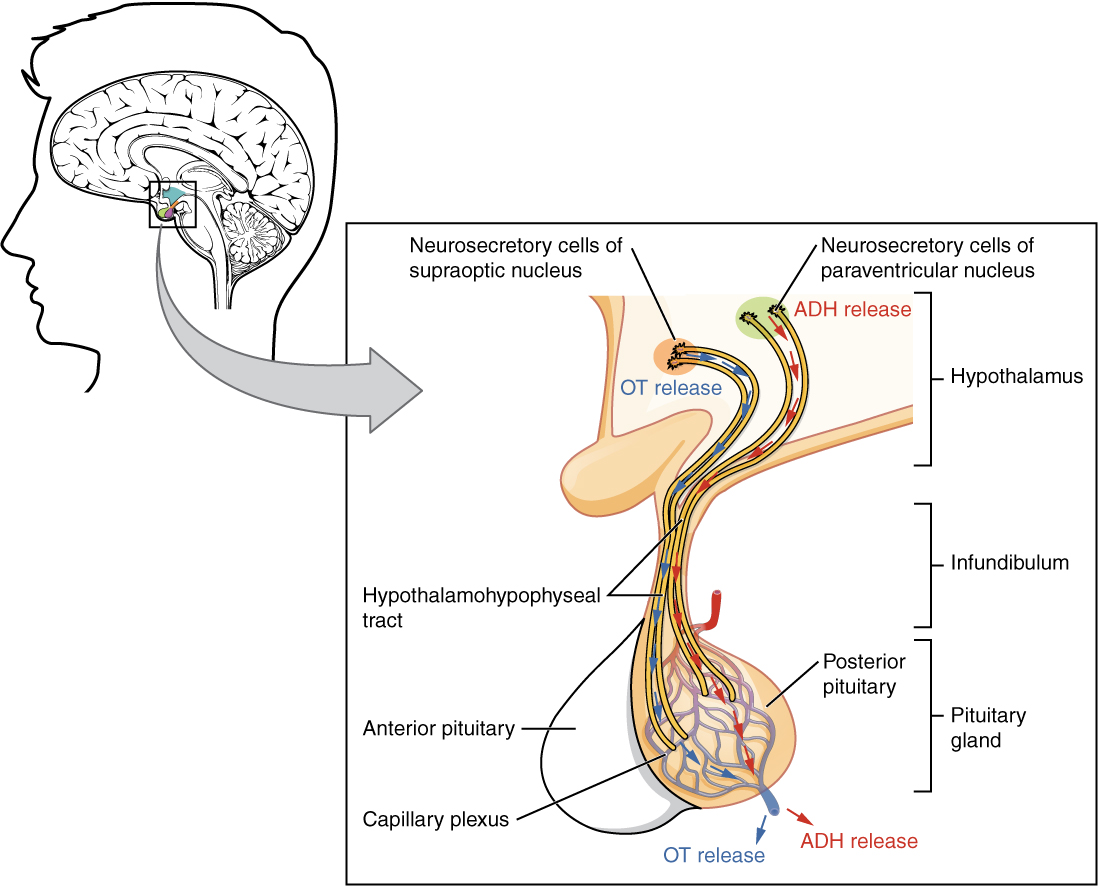
The posterior pituitary gland does not produce hormones, but rather stores and secretes hormones produced by the hypothalamus. The paraventricular nuclei produce the hormone oxytocin, whereas the supraoptic nuclei produce ADH. These hormones travel along the axons into storage sites in the axon terminals of the posterior pituitary. In response to signals from the same hypothalamic neurons, the hormones are released from the axon terminals into the bloodstream.
Oxytocin
When fetal development is complete, the peptide-derived hormone oxytocin (tocia- = “childbirth”) stimulates uterine contractions and dilation of the cervix. Throughout most of pregnancy, oxytocin hormone receptors are not expressed at high levels in the uterus. Toward the end of pregnancy, the synthesis of oxytocin receptors in the uterus increases, and the smooth muscle cells of the uterus become more sensitive to its effects. Oxytocin is continually released throughout childbirth through a positive feedback mechanism. As noted earlier, oxytocin prompts uterine contractions that push the fetal head toward the cervix. In response, cervical stretching stimulates additional oxytocin to be synthesized by the hypothalamus and released from the pituitary. This increases the intensity and effectiveness of uterine contractions and prompts additional dilation of the cervix. The feedback loop continues until birth.
Although the mother’s high blood levels of oxytocin begin to decrease immediately following birth, oxytocin continues to play a role in maternal and newborn health. First, oxytocin is necessary for the milk ejection reflex (commonly referred to as “let-down”) in breastfeeding women. As the newborn begins suckling, sensory receptors in the nipples transmit signals to the hypothalamus. In response, oxytocin is secreted and released into the bloodstream. Within seconds, cells in the mother’s milk ducts contract, ejecting milk into the infant’s mouth. Secondly, in both males and females, oxytocin is thought to contribute to parent–newborn bonding, known as attachment. Oxytocin is also thought to be involved in feelings of love and closeness, as well as in the sexual response.
Antidiuretic Hormone (ADH)
The solute concentration of the blood, or blood osmolarity, may change in response to the consumption of certain foods and fluids, as well as in response to disease, injury, medications, or other factors. Blood osmolarity is constantly monitored by osmoreceptors—specialized cells within the hypothalamus that are particularly sensitive to the concentration of sodium ions and other solutes.
In response to high blood osmolarity, which can occur during dehydration or following a very salty meal, the osmoreceptors signal the posterior pituitary to release antidiuretic hormone (ADH). The target cells of ADH are located in the tubular cells of the kidneys. Its effect is to increase epithelial permeability to water, allowing increased water reabsorption. The more water reabsorbed from the filtrate, the greater the amount of water that is returned to the blood and the less that is excreted in the urine. A greater concentration of water results in a reduced concentration of solutes. ADH is also known as vasopressin because, in very high concentrations, it causes constriction of blood vessels, which increases blood pressure by increasing peripheral resistance. The release of ADH is controlled by a negative feedback loop. As blood osmolarity decreases, the hypothalamic osmoreceptors sense the change and prompt a corresponding decrease in the secretion of ADH. As a result, less water is reabsorbed from the urine filtrate.
Interestingly, drugs can affect the secretion of ADH. For example, alcohol consumption inhibits the release of ADH, resulting in increased urine production that can eventually lead to dehydration and a hangover. A disease called diabetes insipidus is characterized by chronic underproduction of ADH that causes chronic dehydration. Because little ADH is produced and secreted, not enough water is reabsorbed by the kidneys. Although patients feel thirsty, and increase their fluid consumption, this doesn’t effectively decrease the solute concentration in their blood because ADH levels are not high enough to trigger water reabsorption in the kidneys. Electrolyte imbalances can occur in severe cases of diabetes insipidus.
Thyroid-Stimulating Hormone
The activity of the thyroid gland is regulated by thyroid-stimulating hormone (TSH), also called thyrotropin. TSH is released from the anterior pituitary in response to thyrotropin-releasing hormone (TRH) from the hypothalamus. As discussed shortly, it triggers the secretion of thyroid hormones by the thyroid gland. In a classic negative feedback loop, elevated levels of thyroid hormones in the bloodstream then trigger a drop in production of TRH and subsequently TSH.
Adrenocorticotropic Hormone
The adrenocorticotropic hormone (ACTH), also called corticotropin, stimulates the adrenal cortex (the more superficial “bark” of the adrenal glands) to secrete corticosteroid hormones such as cortisol. ACTH come from a precursor molecule known as pro-opiomelanotropin (POMC) which produces several biologically active molecules when cleaved, including ACTH, melanocyte-stimulating hormone, and the brain opioid peptides known as endorphins. The release of ACTH is regulated by the corticotropin-releasing hormone (CRH) from the hypothalamus in response to normal physiologic rhythms. A variety of stressors can also influence its release, and the role of ACTH in the stress response is discussed later in this chapter.
Follicle-Stimulating Hormone and Luteinizing Hormone
The endocrine glands secrete a variety of hormones that control the development and regulation of the reproductive system (these glands include the anterior pituitary, the adrenal cortex, and the gonads—the testes in males and the ovaries in females). Much of the development of the reproductive system occurs during puberty and is marked by the development of sex-specific characteristics in both male and female adolescents. Puberty is initiated by gonadotropin-releasing hormone (GnRH), a hormone produced and secreted by the hypothalamus. GnRH stimulates the anterior pituitary to secrete gonadotropins—hormones that regulate the function of the gonads. The levels of GnRH are regulated through a negative feedback loop; high levels of reproductive hormones inhibit the release of GnRH. Throughout life, gonadotropins regulate reproductive function and, in the case of women, the onset and cessation of reproductive capacity.
The gonadotropins include two glycoprotein hormones: follicle-stimulating hormone (FSH) stimulates the production and maturation of sex cells, or gametes, including ova in women and sperm in men. FSH also promotes follicular growth; these follicles then release estrogens in the female ovaries. Luteinizing hormone (LH) triggers ovulation in women, as well as the production of estrogens and progesterone by the ovaries. LH stimulates production of testosterone by the male testes.
Prolactin
As its name implies, prolactin (PRL) promotes lactation (milk production) in women. During pregnancy, it contributes to development of the mammary glands, and after birth, it stimulates the mammary glands to produce breast milk. However, the effects of prolactin depend heavily upon the permissive effects of estrogens, progesterone, and other hormones. And as noted earlier, the let-down of milk occurs in response to stimulation from oxytocin.
In a non-pregnant woman, prolactin secretion is inhibited by prolactin-inhibiting hormone (PIH), which is actually the neurotransmitter dopamine, and is released from neurons in the hypothalamus. Only during pregnancy do prolactin levels rise in response to prolactin-releasing hormone (PRH) from the hypothalamus.
Intermediate Pituitary: Melanocyte-Stimulating Hormone
The cells in the zone between the pituitary lobes secrete a hormone known as melanocyte-stimulating hormone (MSH) that is formed by cleavage of the pro-opiomelanocortin (POMC) precursor protein. Local production of MSH in the skin is responsible for melanin production in response to UV light exposure. The role of MSH made by the pituitary is more complicated. For instance, people with lighter skin generally have the same amount of MSH as people with darker skin. Nevertheless, this hormone is capable of darkening of the skin by inducing melanin production in the skin’s melanocytes. Women also show increased MSH production during pregnancy; in combination with estrogens, it can lead to darker skin pigmentation, especially the skin of the areolas and labia minora. Table 20.4 is a summary of the pituitary hormones and their principal effects.
| IMAGE OF GLANDS | HORMONES | |||
 |
Posterior Pituitary Hormones | |||
| Releasing hormone (hypothalamus) | Pituitary Hormone | Target | Effects | |
| ADH | Stores ADH | Kidneys, sweat glands, circulatory system | Water balance | |
| – | OT | Female reproductive system | Triggers uterine contractions during childbirth | |
 |
Anterior Pituitary Hormones | |||
| Releasing hormone (hypothalamus) | Pituitary Hormone | Target | Effects | |
| GnRH | LH | Reproductive system | Stimulates production of sex hormones by gonads | |
| GnRH | FSH | Reproductive system | stimulates production of sperm and eggs | |
| TRH | TSH | Thyroid gland | STimulates the release of thyroid hormone (TH), TH regulates metabolism | |
| PRH (inhibited by PIH) | PRL | Mammary glands | Promotes milk production | |
| GHRH (inhibited by GHIH) | GH | Liver, bone, muscles | Induces targets to produce insulin-like growth factors (IGF). IGFs stimulate body growth and higher metabolic rate. | |
| CRH | ACTH | Adrenal glands | Induces targets to produce glucocorticoids, which regulate metabolism and stress response | |
Pineal Gland
A tiny endocrine gland whose functions are not entirely clear. The pinealocyte cells that make up the pineal gland are known to produce and secrete the amine hormone melatonin, which is derived from serotonin.
The secretion of melatonin varies according to the level of light received from the environment. When photons of light stimulate the retinas of the eyes, a nerve impulse is sent to a region of the hypothalamus which is important in regulating biological rhythms. When blood levels of melatonin fall they promote wakefulness. In contrast, as light levels decline—such as during the evening—melatonin production increases, boosting blood levels and causing drowsiness.
Watch this video:
https://www.youtube.com/watch?v=EUyBDGgsk_I
Media 20.2 What Does Melatonin Do? Melatonin Use Info [Online video]. Copyright 2015 by Travelers Defense.
What should you avoid doing in the middle of your sleep cycle that would lower melatonin?
The secretion of melatonin may influence the body’s circadian rhythms, the dark-light fluctuations that affect not only sleepiness and wakefulness, but also appetite and body temperature. Interestingly, children have higher melatonin levels than adults, which may prevent the release of gonadotropins from the anterior pituitary, thereby inhibiting the onset of puberty. Finally, an antioxidant role of melatonin is the subject of current research.
Jet lag occurs when a person travels across several time zones and feels sleepy during the day or wakeful at night. Traveling across multiple time zones significantly disturbs the light-dark cycle regulated by melatonin. It can take up to several days for melatonin synthesis to adjust to the light-dark patterns in the new environment, resulting in jet lag. Some air travelers take melatonin supplements to induce sleep.
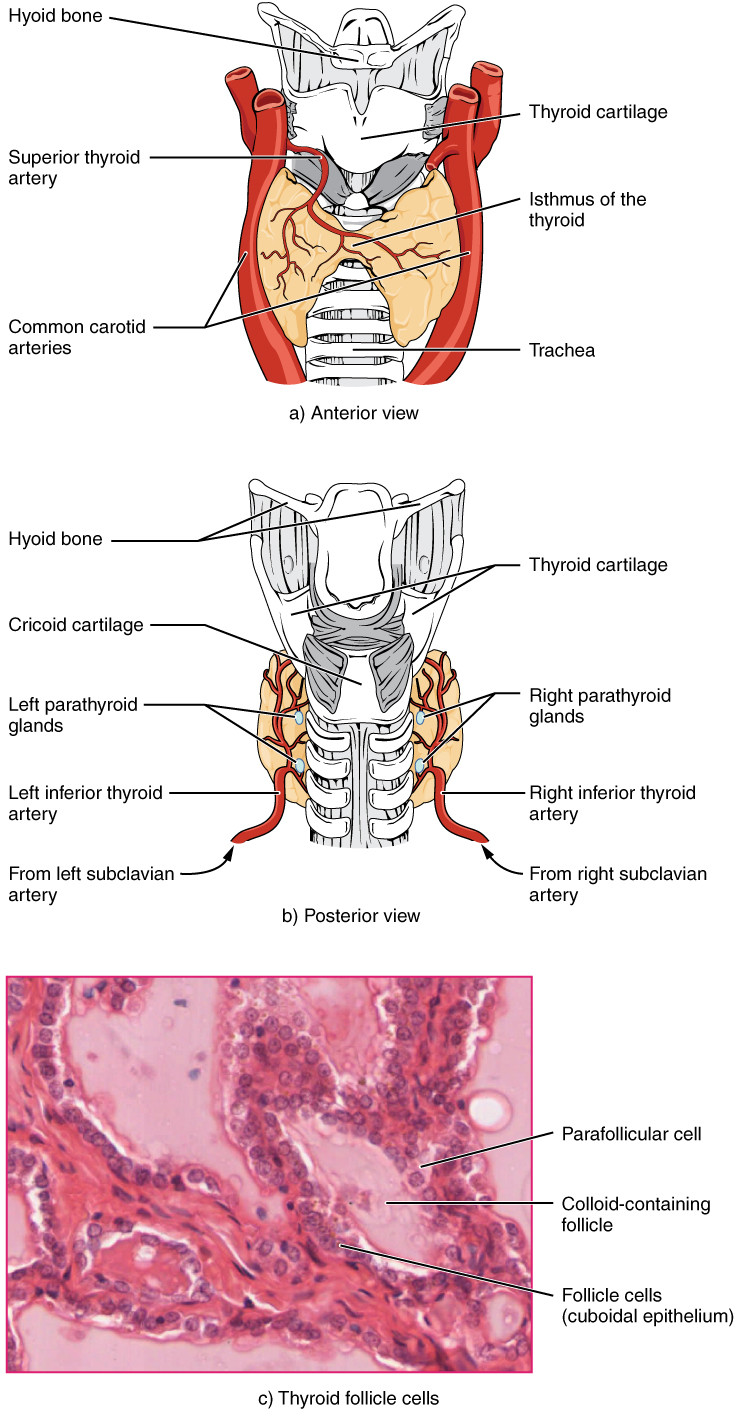
Thyroid Gland
A butterfly-shaped organ, the thyroid gland is located anterior to the trachea, just inferior to the larynx (see Figure 20.7). The medial region, called the isthmus, is flanked by wing-shaped left and right lobes. Each of the thyroid lobes are embedded with parathyroid glands, primarily on their posterior surfaces. The tissue of the thyroid gland is composed mostly of thyroid follicles. The follicles are made up of a central cavity filled with a sticky fluid called colloid. Surrounded by a wall of epithelial follicle cells, the colloid is the center of thyroid hormone production, and that production is dependent on the hormones’ essential and unique component: iodine.
Regulation of TH Synthesis
The release of T3 and T4 from the thyroid gland is regulated by thyroid-stimulating hormone (TSH). Low blood levels of T3 and T4 stimulate the release of thyrotropin-releasing hormone (TRH) from the hypothalamus, which triggers secretion of TSH from the anterior pituitary. In turn, TSH stimulates the thyroid gland to secrete T3 and T4. The levels of TRH, TSH, T3, and T4 are regulated by a negative feedback system in which increasing levels of T3 and T4 decrease the production and secretion of TSH. The thyroid hormones, T3 and T4, are often referred to as metabolic hormones because their levels influence the body’s basal metabolic rate, the amount of energy used by the body at rest.
The thyroid gland also secretes a hormone called calcitonin that is produced by the parafollicular cells (also called C cells) that stud the tissue between distinct follicles. Calcitonin is released in response to a rise in blood calcium levels.
Parathyroid Gland
The parathyroid glands are tiny, round structures usually found embedded in the posterior surface of the thyroid gland. A thick connective tissue capsule separates the glands from the thyroid tissue. Most people have four parathyroid glands, but occasionally there are more in tissues of the neck or chest. The function of one type of parathyroid cells, the oxyphil cells, is not clear. The primary functional cells of the parathyroid glands are the chief cells. These epithelial cells produce and secrete the parathyroid hormone (PTH), the major hormone involved in the regulation of blood calcium levels.
Adrenal Gland
The adrenal glands are wedges of glandular and neuroendocrine tissue adhering to the top of the kidneys by a fibrous capsule (see Figure 20.8). The adrenal glands have a rich blood supply and experience one of the highest rates of blood flow in the body. They are served by several arteries branching off the aorta, including the suprarenal and renal arteries. Blood flows to each adrenal gland at the adrenal cortex and then drains into the adrenal medulla. Adrenal hormones are released into the circulation via the left and right suprarenal veins.
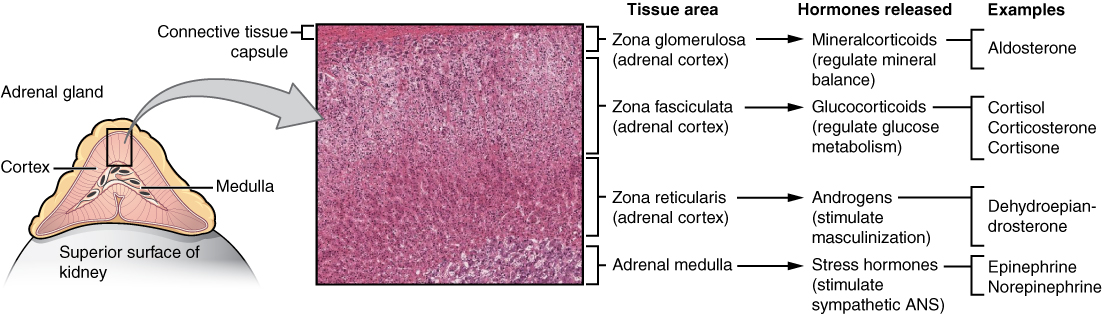
The adrenal cortex, as a component of the hypothalamic-pituitary-adrenal (HPA) axis, secretes steroid hormones important for the regulation of the long-term stress response, blood pressure and blood volume, nutrient uptake and storage, fluid and electrolyte balance, and inflammation. The HPA axis involves the stimulation of hormone release of adrenocorticotropic hormone (ACTH) from the pituitary by the hypothalamus. ACTH then stimulates the adrenal cortex to produce the hormone cortisol. This pathway will be discussed in more detail below.
The adrenal medulla is neuroendocrine tissue composed of postganglionic sympathetic nervous system (SNS) neurons. It is really an extension of the autonomic nervous system, which regulates homeostasis in the body. The sympathomedullary (SAM) pathway involves the stimulation of the medulla by impulses from the hypothalamus via neurons from the thoracic spinal cord. The medulla is stimulated to secrete the amine hormones epinephrine and norepinephrine.
One of the major functions of the adrenal gland is to respond to stress. Stress can be either physical or psychological or both. Physical stresses include exposing the body to injury, walking outside in cold and wet conditions without a coat on, or malnutrition. Psychological stresses include the perception of a physical threat, a fight with a loved one, or just a bad day at school.
The body responds in different ways to short-term stress and long-term stress following a pattern known as the general adaptation syndrome (GAS). Stage one of GAS is called the alarm reaction. This is short-term stress, the fight-or-flight response, mediated by the hormones epinephrine and norepinephrine from the adrenal medulla via the SAM pathway. Their function is to prepare the body for extreme physical exertion. Once this stress is relieved, the body quickly returns to normal. The section on the adrenal medulla covers this response in more detail.
If the stress is not soon relieved, the body adapts to the stress in the second stage called the stage of resistance. If a person is starving for example, the body may send signals to the gastrointestinal tract to maximize the absorption of nutrients from food.
If the stress continues for a longer term however, the body responds with symptoms quite different than the fight-or-flight response. During the stage of exhaustion, individuals may begin to suffer depression, the suppression of their immune response, severe fatigue, or even a fatal heart attack. These symptoms are mediated by the hormones of the adrenal cortex, especially cortisol, released as a result of signals from the HPA axis.
Adrenal hormones also have several non–stress-related functions, including the increase of blood sodium and glucose levels, which will be described in detail below.
Adrenal Cortex
The adrenal cortex consists of multiple layers of lipid-storing cells that occur in three structurally distinct regions. Each of these regions produces different hormones.
Watch this video:
Media 20.3 Endocrine System, Part 2 – Hormone Cascades: Crash Course A&P #24 [Online video]. Copyright 2015 by CrashCourse.
Concept Check
- Which hormone produced by the adrenal glands is responsible for the mobilization of energy stores?
Hormones of the Zona Glomerulosa
The most superficial region of the adrenal cortex is the zona glomerulosa, which produces a group of hormones collectively referred to as mineralocorticoids because of their effect on body minerals, especially sodium and potassium. These hormones are essential for fluid and electrolyte balance.
Aldosterone is the major mineralocorticoid. It is important in the regulation of the concentration of sodium and potassium ions in urine, sweat, and saliva. For example, it is released in response to elevated blood K+, low blood Na+, low blood pressure, or low blood volume. In response, aldosterone increases the excretion of K+ and the retention of Na+, which in turn increases blood volume and blood pressure. Its secretion is prompted when CRH from the hypothalamus triggers ACTH release from the anterior pituitary.
Aldosterone is also a key component of the renin-angiotensin-aldosterone system (RAAS) in which specialized cells of the kidneys secrete the enzyme renin in response to low blood volume or low blood pressure. Renin then catalyzes the conversion of the blood protein angiotensinogen, produced by the liver, to the hormone angiotensin I. Angiotensin I is converted in the lungs to angiotensin II by angiotensin-converting enzyme (ACE). Angiotensin II has three major functions:
- Initiating vasoconstriction of the arterioles, decreasing blood flow
- Stimulating kidney tubules to reabsorb NaCl and water, increasing blood volume
- Signaling the adrenal cortex to secrete aldosterone, the effects of which further contribute to fluid retention, restoring blood pressure and blood volume
For individuals with hypertension, or high blood pressure, drugs are available that block the production of angiotensin II. These drugs, known as ACE inhibitors, block the ACE enzyme from converting angiotensin I to angiotensin II, thus mitigating the latter’s ability to increase blood pressure.
Hormones of the Zona Fasciculata
The intermediate region of the adrenal cortex is the zona fasciculata, named as such because the cells form small fascicles (bundles) separated by tiny blood vessels. The cells of the zona fasciculata produce hormones called glucocorticoids because of their role in glucose metabolism. The most important of these is cortisol, some of which the liver converts to cortisone. A glucocorticoid produced in much smaller amounts is corticosterone. In response to long-term stressors, the hypothalamus secretes CRH, which in turn triggers the release of ACTH by the anterior pituitary. ACTH triggers the release of the glucocorticoids. Their overall effect is to inhibit tissue building while stimulating the breakdown of stored nutrients to maintain adequate fuel supplies. In conditions of long-term stress, for example, cortisol promotes the catabolism of glycogen to glucose, the catabolism of stored triglycerides into fatty acids and glycerol, and the catabolism of muscle proteins into amino acids. These raw materials can then be used to synthesize additional glucose and ketones for use as body fuels. The hippocampus, which is part of the temporal lobe of the cerebral cortices and important in memory formation, is highly sensitive to stress levels because of its many glucocorticoid receptors.
You are probably familiar with prescription and over-the-counter medications containing glucocorticoids, such as cortisone injections into inflamed joints, prednisone tablets and steroid-based inhalers used to manage severe asthma, and hydrocortisone creams applied to relieve itchy skin rashes. These drugs reflect another role of cortisol—the downregulation of the immune system, which inhibits the inflammatory response.
Hormones of the Zona Reticularis
The deepest region of the adrenal cortex is the zona reticularis, which produces small amounts of a class of steroid sex hormones called androgens. During puberty and most of adulthood, androgens are produced in the gonads. The androgens produced in the zona reticularis supplement the gonadal androgens. They are produced in response to ACTH from the anterior pituitary and are converted in the tissues to testosterone or estrogens. In adult women, they may contribute to the sex drive, but their function in adult men is not well understood. In post-menopausal women, as the functions of the ovaries decline, the main source of estrogens becomes the androgens produced by the zona reticularis.
Adrenal Medulla
The medullary tissue is composed of unique postganglionic SNS neurons called chromaffin cells, which are large and irregularly shaped, and produce the neurotransmitters epinephrine (also called adrenaline) and norepinephrine (or noradrenaline). Epinephrine is produced in greater quantities—approximately a 4 to 1 ratio with norepinephrine—and is the more powerful hormone. Because the chromaffin cells release epinephrine and norepinephrine into the systemic circulation, where they travel widely and exert effects on distant cells, they are considered hormones. Derived from the amino acid tyrosine, they are chemically classified as catecholamines.
The secretion of medullary epinephrine and norepinephrine is controlled by a neural pathway that originates from the hypothalamus in response to danger or stress (the SAM pathway). Both epinephrine and norepinephrine signal the liver and skeletal muscle cells to convert glycogen into glucose, resulting in increased blood glucose levels. These hormones increase the heart rate, pulse, and blood pressure to prepare the body to fight the perceived threat or flee from it. In addition, the pathway dilates the airways, raising blood oxygen levels. It also prompts vasodilation, further increasing the oxygenation of important organs such as the lungs, brain, heart, and skeletal muscle. At the same time, it triggers vasoconstriction to blood vessels serving less essential organs such as the gastrointestinal tract, kidneys, and skin, and downregulates some components of the immune system. Other effects include a dry mouth, loss of appetite, pupil dilation, and a loss of peripheral vision.
Pancreas
The pancreas is a long, slender organ, most of which is located posterior to the bottom half of the stomach (see Figure 20.9). Although it is primarily an exocrine gland, secreting a variety of digestive enzymes, the pancreas has an endocrine function. Its pancreatic islets—clusters of cells formerly known as the islets of Langerhans—secrete the hormones glucagon, insulin, somatostatin, and pancreatic polypeptide (PP).
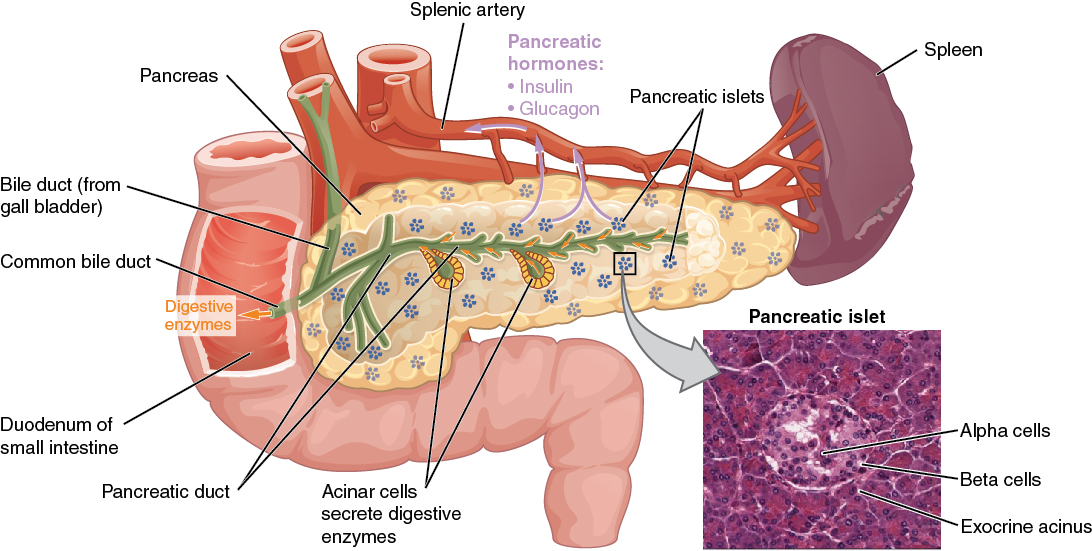
Cells and Secretions of the Pancreatic Islets
The pancreatic islets each contain four varieties of cells:
- The alpha cell produces the hormone glucagon and makes up approximately 20 percent of each islet. Glucagon plays an important role in blood glucose regulation; low blood glucose levels stimulate its release.
- The beta cell produces the hormone insulin and makes up approximately 75 percent of each islet. Elevated blood glucose levels stimulate the release of insulin.
- The delta cell accounts for four percent of the islet cells and secretes the peptide hormone somatostatin. Recall that somatostatin is also released by the hypothalamus (as GHIH), and the stomach and intestines also secrete it. An inhibiting hormone, pancreatic somatostatin inhibits the release of both glucagon and insulin.
- The PP cell accounts for about one percent of islet cells and secretes the pancreatic polypeptide hormone. It is thought to play a role in appetite, as well as in the regulation of pancreatic exocrine and endocrine secretions. Pancreatic polypeptide released following a meal may reduce further food consumption; however, it is also released in response to fasting.
Regulation of Blood Glucose Levels by Insulin and Glucagon
Glucose is required for cellular respiration and is the preferred fuel for all body cells. The body derives glucose from the breakdown of the carbohydrate-containing foods and drinks we consume. Glucose not immediately taken up by cells for fuel can be stored by the liver and muscles as glycogen, or converted to triglycerides and stored in the adipose tissue. Hormones regulate both the storage and the utilization of glucose as required. Receptors located in the pancreas sense blood glucose levels, and subsequently the pancreatic cells secrete glucagon or insulin to maintain normal levels.
Gonadal Glands
The male testes and female ovaries—which produce the sex cells (sperm and ova) and secrete the gonadal hormones. The roles of the gonadotropins released from the anterior pituitary (FSH and LH) were discussed earlier.
The primary hormone produced by the male testes is testosterone, a steroid hormone important in the development of the male reproductive system, the maturation of sperm cells, and the development of male secondary sex characteristics such as a deepened voice, body hair, and increased muscle mass. Interestingly, testosterone is also produced in the female ovaries, but at a much reduced level. In addition, the testes produce the peptide hormone inhibin, which inhibits the secretion of FSH from the anterior pituitary gland. FSH stimulates spermatogenesis.
The primary hormones produced by the ovaries are estrogens, which include estradiol, estriol, and estrone. Estrogens play an important role in a larger number of physiological processes, including the development of the female reproductive system, regulation of the menstrual cycle, the development of female secondary sex characteristics such as increased adipose tissue and the development of breast tissue, and the maintenance of pregnancy. Another significant ovarian hormone is progesterone, which contributes to regulation of the menstrual cycle and is important in preparing the body for pregnancy as well as maintaining pregnancy. In addition, the granulosa cells of the ovarian follicles produce inhibin, which—as in males—inhibits the secretion of FSH. During the initial stages of pregnancy, an organ called the placenta develops within the uterus. The placenta supplies oxygen and nutrients to the fetus, excretes waste products, and produces and secretes estrogens and progesterone. The placenta produces human chorionic gonadotropin (hCG) as well. The hCG hormone promotes progesterone synthesis and reduces the mother’s immune function to protect the fetus from immune rejection. It also secretes human placental lactogen (hPL), which plays a role in preparing the breasts for lactation, and relaxin, which is thought to help soften and widen the pubic symphysis in preparation for childbirth.
Common Endocrine System Abbreviations
Concept Check
- Do you recall the term which describes high level of glucose in the blood?
- Do you recall the neurotransmitter responsible for assisting the response to danger or stress?
- Suggest what may happen if the adrenal cortex failed to secrete its hormones.
Diseases and Disorders
Acromegaly
- A disorder in adults caused when abnormally high levels of GH trigger growth of bones in the face, hands, and feet.
Addison’s disease
A rare disorder that causes low blood glucose levels and low blood sodium levels. The signs and symptoms of Addison’s disease are vague and are typical of other disorders as well, making diagnosis difficult. They may include general weakness, abdominal pain, weight loss, nausea, vomiting, sweating, and cravings for salty food (Betts, et al., 2013).
Cushing’s disease
A disorder characterized by high blood glucose levels and the accumulation of lipid deposits on the face and neck. It is caused by hypersecretion of cortisol. The most common source of Cushing’s disease is a pituitary tumor that secretes cortisol or ACTH in abnormally high amounts (Betts, et al., 2013).
Gigantism
- A disorder in children caused when abnormally high levels of GH prompt excessive growth in the body (Betts, et al., 2013).
Hirsutism
Hirsuitism is a symptom of an excessive production of androgens causing hair growth in women where they typically do not have hair growth. While some medications may cause the increased androgen production it can also be linked to endocrine disorders such as Polycystic Ovary Syndrome (PCOS), Cushing syndrome and tumours in the ovaries or adrenal glands (Mayo Clinic Staff, 2020).
Hyperthyroidism
A condition marked by high levels of thyroid hormones that results in weight loss, profuse sweating, and increased heart rate (Betts, et al., 2013).
Hypothyroidism
A condition marked by low levels of thyroid hormones that results in weight gain, cold sensitivity, and reduced mental activity (Betts, et al., 2013).
Graves Disease
A condition marked by a disorder of the thyroid gland, resulting in hyperthyroidism (Betts, et al., 2013).
Diabetes Inspidius
A condition caused by a lack of or hyposecretion of the antidiuretic hormone (ADH). The condition can also be caused by the failure of the kidneys to respond to ADH (Betts, et al., 2013).
Diabetes (Mellitus)
A condition marked by a disorder of the pancreas, resulting in high levels of glucose in the blood (Betts, et al., 2013).
Medical Terms in Context
Medical Specialties and Procedures Related to the Endocrine System
Endocrinology is a specialization in the field of medicine that focuses on the treatment of endocrine system disorders. Endocrinologists—medical doctors who specialize in this field—are experts in treating diseases associated with hormonal systems, ranging from thyroid disease to diabetes. Endocrine surgeons treat endocrine disease through the removal of the affected endocrine gland or tissue. Some patients experience health problems as a result of the normal decline in hormones that can accompany aging. These patients can consult with an endocrinologist to weigh the risks and benefits of hormone replacement therapy intended to boost their natural levels of reproductive hormones. In addition to treating patients, endocrinologists may be involved in research to improve the understanding of endocrine system disorders and develop new treatments for these diseases (Betts, et al., 2013).
- A thyroid specialist is an endocrinologist whose sub specialty is focused on the treatment and disorders of the thyroid gland such as hypothyroidism (too low secretion) and hyperthyroidism (too high secretion).
- A diabetes specialist is an endocrinologist whose sub specialty is focused on the treatment of diabetic conditions.
Procedures
Thyroid Scan
This procedure is designed to check the status of the thyroid. In a thyroid scan, a radioactive compound is given and localized in the thyroid gland (Giorgi & Cherney, 2018). To learn more about a thyroid scan visit HealthLine: Thyroid Scan.
Radioactive iodine uptake
Thyroid function evaluated by injecting radioactive iodine and then measuring how much is removed from the blood by the thyroid (MedlinePlus, 2020). To learn more about a radioactive iodine update test visit Medline Plus: Radioactive Iodine Uptake.
Blood Serum Testing
Blood testing to determine the concentration and the presence of various endocrine hormones in the blood. These tests include the following levels: calcium, cortisol, electrolytes, FSH, GH, glucose, insulin, parathyroid hormones, T3, T4, testosterone, and TSH. All of these can be evaluated with blood serum tests (Betts, et al., 2013).
Endocrine Surgical Procedures
Most of the surgeries and procedures performed with the endocrine system involve removal of a gland or an incision into the gland. Once an endocrine gland is surgically removed, due to a tumor or enlargement, hormone replacement treatment is required. Medication is required to artificially or synthetically replace the hormone produced by the gland and the function it regulates (Betts, et al., 2013).
Endocrine System Vocabulary
- Autocrine
Chemical signal that elicits a response in the same cell that secreted it.
Endocrine gland
Tissue or organ that secretes hormones into the blood and lymph without ducts such that they may be transported to organs distant from the site of secretion.
Endocrine system
Cells, tissues, and organs that secrete hormones as a primary or secondary function and play an integral role in normal bodily processes.
Epinephrine
Also known as adrenaline, is a hormone and neurotransmitter and produced by the adrenal glands.
Exocrine system
Cells, tissues, and organs that secrete substances directly to target tissues via glandular ducts.
Histamine
Involved in the inflammatory response and typically causes itching.
Hormone
Secretion of an endocrine organ that travels via the bloodstream or lymphatics to induce a response in target cells or tissues in another part of the body.
Neurotransmitter
Chemicals acting as signaling molecules that enable neurotransmission.
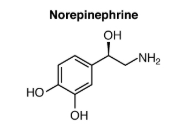
Norepinephrine
A natural chemical in the body that acts as both a stress hormone and neurotransmitter (a substance that sends signals between nerve cells). It’s released into the blood as a stress hormone when the brain perceives stress.
Paracrine
Chemical signal that elicits a response in neighbouring cells; also called paracrine factor.
Permeability
Membrane that causes it to allow liquids or gases to pass through it.
Proliferation
Rapid increase in numbers.
Synthesis
The production of chemical compounds by reaction from simpler materials.
Test Yourself
References
[CrashCourse]. (2015, June 22). Endocrine system, part 1 – glands & hormones: Crash course A&P #23 [Video]. YouTube. https://www.youtube.com/watch?v=eWHH9je2zG4
[CrashCourse]. (2015, June 29). Endocrine system, part 2 – hormone cascade: Crash course A&P #24 [Video]. YouTube. https://www.youtube.com/watch?v=eWHH9je2zG4
Giorgi, A., & Cherney, K. (2018). Thyroid scan. Healthline. https://www.healthline.com/health/thyroid-scan
MedlinePlus. (2020). Radioactive iodine uptake. US National Library of Medicine. https://medlineplus.gov/ency/article/003689.htm
Shurkin, J.N.(2013, August 2). Trouble sleeping? Go camping: Artificial light sources can negatively affect circadian rhythms, scientists say. Scientific American. https://www.scientificamerican.com/article/trouble-sleeping-go-campi/
[TravelersDefense]. (2009, July 28). What does melatonin do? Melatonin use info [Video]. YouTube. https://www.youtube.com/watch?v=EUyBDGgsk_I
Image Descriptions
Figure 20.1 image description: This photo shows a young girl reaching for an orange leaf on an oak tree. She is on a walkway near a creek. The opposite shore is a deep slope covered with more trees in autumn colors. [Return to Figure 20.1].
Figure 20.2 image description: This diagram shows the endocrine glands and cells that are located throughout the body. The endocrine system organs include the pineal gland and pituitary gland in the brain. The pituitary is located on the anterior side of the thalamus while the pineal gland is located on the posterior side of the thalamus. The thyroid gland is a butterfly-shaped gland that wraps around the trachea within the neck. Four small, disc-shaped parathyroid glands are embedded into the posterior side of the thyroid. The adrenal glands are located on top of the kidneys. The pancreas is located at the center of the abdomen. In females, the two ovaries are connected to the uterus by two long, curved, tubes in the pelvic region. In males, the two testes are located in the scrotum below the penis. [Return to Figure 20.2].
Figure 20.3 image description: This diagram shows a negative feedback loop using the example of glucocorticoid regulation in the blood. Step 1 in the cycle is when an imbalance occurs. The hypothalamus perceives low blood concentrations of glucocorticoids in the blood. This is illustrated by there being only 5 glucocorticoids floating in a cross section of an artery. Step 2 in the cycle is hormone release, where the hypothalamus releases corticotropin-releasing hormone (CRH). Step 3 is labeled correction. Here, the CRH release starts a hormone cascade that triggers the adrenal gland to release glucocorticoid into the blood. This allows the blood concentration of glucocorticoid to increase, as illustrated by 8 glucocorticoid molecules now being present in the cross section of the artery. Step 4 is labeled negative feedback. Here, the hypothalamus perceives normal concentrations of glucocorticoids in the blood and stops releasing CRH. This brings blood glucocorticoid levels back to homeostasis. [Return to Figure 20.3].
Figure 20.4 image description: This illustration zooms in on the hypothalamus and the attached pituitary gland. The anterior pituitary is highlighted. Three neurosecretory cells are secreting hormones into a web-like network of arteries within the infundibulum. The artery net is labeled the primary capillary plexus of the hypophyseal portal system. The superior hypophysel artery enters the primary capillary plexus from outside of the infundibulum. The hypophyseal portal vein runs down from the primary capillary plexus, through the infundibulum, and connects to the secondary capillary plexus of the hypophyseal portal system. The secondary capillary plexus is located within the anterior pituitary. The hormones released from the neurosecretory cells of the hypothalamus travel through the primary capillary plexus, down the hypophyseal portal vein, and into the secondary capillary plexus. There, the hypothalamus hormones stimulate the anterior pituitary to release its hormones. The anterior pituitary hormones leave the primary capillary plexus from a single vein at the bottom of the anterior lobe. [Return to Figure 20.4].
Figure 20.5 image description: This flow chart illustrates the hormone cascade that stimulates human growth. In step 1, the hypothalamus releases growth hormone-releasing hormone (GHRH). GHRH travels into the primary capillary plexus of the anterior pituitary, where it stimulates the anterior pituitary to release growth hormone (GH). The release of growth hormone causes three types of effects. In the glucose-sparing effect, GH stimulates adipose cells to break down stored fat, fueling the growth effects (discussed next). The target cells for the glucose-sparing effects are adipose cells. In the growth effects, GH increases the uptake of amino acids from the blood and enhances cellular proliferation while also reducing apoptosis. The target cells for the growth effects are bone cells, muscle cells, nervous system cells, and immune system cells. In the diabetogenic effect, GH stimulates the liver to break down glycogen into glucose, fueling the growth effects. The liver also releases IGF in response to GH. The IGF further stimulates the growth effects but also negatively feeds back to the hypothalamus. When high IGF one levels are perceived by the hypothalamus, it releases growth hormone inhibiting hormone (GHIH). GHIH inhibits GH release by the anterior pituitary. [Return to Figure 20.5].
Figure 20.6 image description: This illustration zooms in on the hypothalamus and the attached pituitary gland. The posterior pituitary is highlighted. Two nuclei in the hypothalamus contain neurosecretory cells that release different hormones. The neurosecretory cells of the paraventricular nucleus release oxytocin (OT) while the neurosecretory cells of the supraoptic nucleus release anti-diuretic hormone (ADH). The neurosecretory cells stretch down the infundibulum into the posterior pituitary. The tube-like extensions of the neurosecretory cells within the infundibulum are labeled the hypothalamophypophyseal tracts. These tracts connect with a web-like network of blood vessels in the posterior pituitary called the capillary plexus. From the capillary plexus, the posterior pituitary secretes the OT or ADH into a single vein that exits the pituitary. [Return to Figure 20.6].
Figure 20.7 image description: Part A of this figure is a diagram of the anterior view of the thyroid gland. The thyroid gland is a butterfly-shaped gland wrapping around the trachea. It narrows at its center, just under the thyroid cartilage of the larynx. This narrow area is called the isthmus of the thyroid. Two large arteries, the common carotid arteries, run parallel to the trachea on the outer border of the thyroid. A small artery enters the superior edge of the thyroid, near the isthmus, and branches throughout the two “wings” of the thyroid. Part B of this figure is a posterior view of the thyroid. The posterior view shows that the thyroid does not completely wrap around the posterior of the trachea. The posterior sides of the thyroid wings can be seen protruding from under the cricoid cartilage of the larynx. The posterior sides of the thyroid “wings” each contain two small, disc-shaped parathyroid glands embedded in the thyroid tissue. Within each wing, one disc is located superior to the other. These are labeled the left and right parathyroid glands. Just under the inferior parathyroid glands are two arteries that bring blood to the thyroid from the left and right subclavian arteries. Part C of this figure is a micrograph of thyroid tissue. The thyroid follicle cells are cuboidal epithelial cells. These cells form a ring around irregular-shaped cavities called follicles. The follicles contain light colored colloid. A larger parafollicular cell is embedded between two of the follicular cells near the edge of a follicle. [Return to Figure 20.7].
Figure 20.8 image description: This diagram shows the left adrenal gland located atop the left kidney. The gland is composed of an outer cortex and an inner medulla all surrounded by a connective tissue capsule. The cortex can be subdivided into additional zones, all of which produce different types of hormones. The outermost layer is the zona glomerulosa, which releases mineralcorticoids, such as aldosterone, that regulate mineral balance. Underneath this layer is the zona fasciculate, which releases glucocorticoids, such as cortisol, corticosterone and cortisone, that regulate glucose metabolism. Underneath this layer is the zona reticularis, which releases androgens, such as dehydroepiandrosterone, that stimulate masculinization. Below this layer is the adrenal medulla, which releases stress hormones, such as epinephrine and norepinephrine, that stimulate the symphathetic ANS. [Return to Figure 20.8].
Figure 20.9 image description: This diagram shows the anatomy of the pancreas. The left, larger side of the pancreas is seated within the curve of the duodenum of the small intestine. The smaller, rightmost tip of the pancreas is located near the spleen. The splenic artery is seen travelling to the spleen, however, it has several branches connecting to the pancreas. An interior view of the pancreas shows that the pancreatic duct is a large tube running through the center of the pancreas. It branches throughout its length in to several horseshoe- shaped pockets of acinar cells. These cells secrete digestive enzymes, which travel down the bile duct and into the small intestine. There are also small pancreatic islets scattered throughout the pancreas. The pancreatic islets secrete the pancreatic hormones insulin and glucagon into the splenic artery. An inset micrograph shows that the pancreatic islets are small discs of tissue consisting of a thin, outer ring called the exocrine acinus, a thicker, inner ring of beta cells and a central circle of alpha cells. [Return to Figure 20.9].
Unless otherwise indicated, this chapter contains material adapted from Anatomy and Physiology (on OpenStax), by Betts, et al. and is used under a a CC BY 4.0 international license. Download and access this book for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction.
also known as adrenaline, is a hormone and neurotransmitter and produced by the adrenal glands
A natural chemical in the body that acts as both a stress hormone and neurotransmitter (a substance that sends signals between nerve cells). It's released into the blood as a stress hormone when the brain perceives stress.
a cell secretes a hormone or chemical messenger.
relating to or denoting a hormone which has effect only in the vicinity of the gland secreting it
Involved in the inflammatory response and typically causes itching.
the production of chemical compounds by reaction from simpler materials.
membrane that causes it to allow liquids or gases to pass through it.
rapid increase in numbers.
chemicals acting as signaling molecules that enable neurotransmission.
male hormones

