ENZYMES
LEARNING OBJECTIVES
Define enzyme and substrate.
Explain how enzymes increase the rate of a chemical reaction.
Determine if enzyme concentration, substrate concentration, pH, and temperature effect the activity of catalase.
MCCCCD OFFICIAL COURSE COMPETENCIES
Describe and utilize the process of scientific inquiry, its realm, and limitations.
Describe structural characteristics of the major groups of microorganisms.
Explain and demonstrate the mechanisms of microbial growth and metabolism.
NOTES
Prepare the yeast solution and wait 30 minutes before you begin the experiments
PHOTO REQUIREMENTS
Take a photo with your photo ID during the lab exercise when you see this icon.

Paste the photos into the Enzymes Questions Document.
Relevant Test Chapters:
introduction
When you light your backyard barbecue, why do you add lighter fluid? You add the lighter fluid to decrease the time it takes for the coals to get hot. In this lab we will investigate enzymes, the lighter fluid of microbial metabolism. Enzymes are usually made of protein, but they can be made of other substances like RNA. Catalase is a protein enzyme that breaks down hydrogen peroxide (H2O2) to oxygen (O2) and water (H2O). One molecule of catalase can breakdown 40,000,000 molecules of H2O2 per second! This enzyme is important is bacterial metabolism because the hydrogen peroxide produced during aerobic energy production must be broken down or it will accumulate and kill the bacteria.
Enzymes are biological catalysts. What is a catalyst? A catalyst is something that increases the rate of a chemical reaction. Here is an example: let’s say our chemical reaction is A + B -> AB. Here is how an enzyme works. First, enzymes will only catalyze reactions that would happen without them. So A + B -> AB is going to happen whether an enzyme is involved or not, it is just going to happen faster with the enzyme. Second, enzymes are not consumed in the process of catalysis. Enzymes catalyze a reaction and then go on to catalyze more, you can’t use them up.
H2O2 + Catalase -> H20 + O2 + Catalase
Why is catalase listed again on the product side of the chemical reaction? Enzymes are not consumed in the process of catalysis
Third, enzymes are very specific, they usually only catalyze one reaction. So our enzyme that catalyzes A + B -> AB would not catalyze C + D -> CD.
The substrate is the molecule the enzyme acts on; they bind together to form an enzyme-substrate complex. The product is what is produced by the chemical reaction. Does the entire catalase molecule bind to hydrogen peroxide? No, actually it doesn’t. The active site is the site on an enzyme where catalysis takes place, the point where the substrate binds. The interaction is often described like a lock and a key. Does your car key open the door lock to your house? No, there is that specificity again.
How do enzymes increase the rate of a chemical reaction? They decrease the activation energy. The activation energy is the energy required for a chemical reaction to proceed. What can affect enzyme activity? Since enzymes are usually proteins, anything that changes the 3-D shape of the protein can possibly affect its activity.
We will use a yeast suspension as our source of catalase (enzyme). Hydrogen peroxide will be the substrate. We will estimate the rate of reaction (or how quickly catalase breaks apart hydrogen peroxide into water and oxygen) by observing bubble (oxygen) formation.
SCIENTIFIC METHOD
Question-What affects the activity of catalase?
Hypothesis-Enzyme and substrate concentration, pH, and temperature affect the activity of catalase.
Experimentation-You will vary enzyme concentration, substrate concentration, pH, and temperature. You will observe bubble (oxygen) formation.
enzyme procedure
| REQUIRED SUPPLIES FROM THE LAB KIT |
| Sterile Transfer Pipettes (6
|
| Test Tubes (4)
|
| Test Tube Rack
|
| Plastic Cups (5)
|
| 100 ml Graduated Cylinder
|
| Test Tube Brush
|
| REQUIRED SUPPLIES YOU PROVIDE |
| Yeast (1 packet ; there is 2 1/4 teaspoon of yeast per packet) |
| Hydrogen peroxide |
| Water (tap is fine) |
| White distilled vinegar |
| Isopropyl alcohol |
| Cup or bowl for surface disinfectant |
| Bleach |
| Ice |
| Sugar (white, 1 teaspoon) |
| Measuring cup |
| Measuring spoons |
| Microwave and microwave safe cup or bowl OR stove and pot |
| Permanent marker |
| Paper towel |
| Dish soap |
| Spoon |
1. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.
2. Prepare surface disinfectant. Using the 100 ml graduated cylinder add 180 ml of tap water to a cup or bowl. Using the 100 ml graduated cylinder now add 20 ml of bleach to the cup or bowl. The surface disinfectant (10% bleach solution) is now ready to use.
3. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash.
prepare the catalase enzyme (yeast solution)
1. Use the permanent marker to label one plastic cup enzyme.
2. Add one packet of yeast (2 and 1/4 teaspoons) to the cup.
3. Add 1/4 cup of very warm tap water to the cup. Use water that is warm, as if you were going to take a shower. If the water is too warm, it will kill the yeast. If the water is not warm enough, it will slow the activation of the yeast. Stir the yeast and water with a spoon.
4. Add 1 teaspoon of white sugar to the cup. Stir with a spoon.
5. Let the enzyme sit at room temperature for 30 minutes.
prepare the ice cold enzyme
1. After the enzyme has sit at room temperature for 30 minutes, prepare an ice bath.
2. Use the permanent marker to label one tube ice. Put ice and water in a plastic cup from the lab kit.
3. Use a sterile transfer pipette to add 2 ml of enzyme to the tube you labeled ice. There are markings on the stem of the pipette to help you measure 2 ml. The full stem of the pipette is 1 ml. Leave the transfer pipette in the enzyme so you can pipette enzyme for the duration of the lab exercise.
4. Place the ice test tube in the ice bath to chill the enzyme. We will be using the ice cold enzyme in the temperature experiment. Set it aside for now.
effect of enzyme (catalase) concentration experiment
To study the effect of enzyme concentration on enzyme activity you will prepare several dilutions of the catalase enzyme.
1. Use the permanent marker to label one test tube 1, one test tube 2, and one test tube 3. Using the pipette in the enzyme add 2 ml of enzyme to test tube 1, add 1 ml of enzyme to test tube 2, and do not add enzyme to test tube 3. Remember there is a mark on the stem of the pipette for 1 ml. Test tube 1 contains 100% enzyme, test tube 2 contains 50% enzyme, and test tube 3 contains 0% enzyme.
2. Use the permanent marker to label a plastic cup from the lab kit water. Add about one inch of tap water to the plastic cup. Using a new sterile, transfer pipette add 1 ml of water to test tube 2 and 2 ml of water to test tube 3. Dispose of the water cup and transfer pipette used for the water in the trash.
3. Use the permanent marker to label a plastic cup from the lab kit hydrogen peroxide. Add about one inch of hydrogen peroxide (H2O2)to the plastic cup. Use a new sterile, transfer pipette and add 2 ml of H2O2 to all 3 test tubes. Leave the pipette in the hydrogen peroxide so you can use the pipette to pipette hydrogen peroxide for the duration of the lab exercise.
4. Observe the bubbles (oxygen) produced in each test tube. Use the scale of 0-3 to describe the rate of reaction and record your results in the data table in the Enzymes Question Document.
0=no reaction (no bubbles formed)
1 = few bubbles formed
2 = some bubbles formed
3 = many bubbles formed
5.  Take a photo with your photo ID of the test tubes in the test tube rack.
Take a photo with your photo ID of the test tubes in the test tube rack.
6. Clean the test tube thoroughly with the test tube brush and soap and water.

effect of substrate (hydrogen peroxide) concentration experiment
To study the effect of substrate concentration on catalase activity you will make dilutions of the substrate (hydrogen peroxide).
1. Using the transfer pipette in the H2O2 add 2 ml of H2O2 to test tube 1, add 1 ml of H2O2 to test tube 2, and do not add H2O2 to test tube 3. Test tube 1 will contains 3% H2O2, test tube 2 contains 1.5% H2O2, and test tube 3 contains 0% H2O2.
2. Using the pipette in the enzyme add 2 ml of enzyme to all 3 test tubes.
4. Observe the bubbles (oxygen) produced in each test tube. Use the scale of 0-3 to describe the rate of reaction and record your results in the data table in the Enzymes Question Document.
0=no reaction (no bubbles formed)
1 = few bubbles formed
2 = some bubbles formed
3 = many bubbles formed
5.  Take a photo with your photo ID of the test tubes in the test tube rack.
Take a photo with your photo ID of the test tubes in the test tube rack.
6. Clean the test tube thoroughly with the test tube brush and soap and water.

effect of pH on catalase activity experiment
You are going to change the pH of the enzyme solution for this experiment.
1. Using the pipette in the enzyme add 2 ml of enzyme into three test tubes.
2. You will not add anything to alter the pH of test tube number 1. The enzyme solution has a pH near neutral of about 6.
3. Using a new sterile transfer pipette add 2 ml of white distilled vinegar to test tube number 2. Mix.
4. Using a new sterile transfer pipette add 2 ml of bleach to test tube number 3. Mix.
5. Let the three test tubes sit for 10 minutes. The enzyme solution has a pH near neutral of about 6. The enzyme solution you added vinegar to will have an acidic pH of about 3. The enzyme solution you added the bleach to will have a basic pH of about 8.
6. After 10 minutes using the transfer pipette in the H2O2 add 2 ml of H2O2 to all 3 test tubes.
7. Observe the bubbles (oxygen) produced in each test tube. Use the scale of 0-3 to describe the rate of reaction and record your results in the data table in the Enzymes Question Document.
0=no reaction (no bubbles formed)
1 = few bubbles formed
2 = some bubbles formed
3 = many bubbles formed
8.  Take a photo with your photo ID of the test tubes in the test tube rack.
Take a photo with your photo ID of the test tubes in the test tube rack.
9. Clean the test tube thoroughly with the test tube brush and soap and water. Remove the 1, 2, and 3 labels from the test tubes using paper towel and isopropyl alcohol.

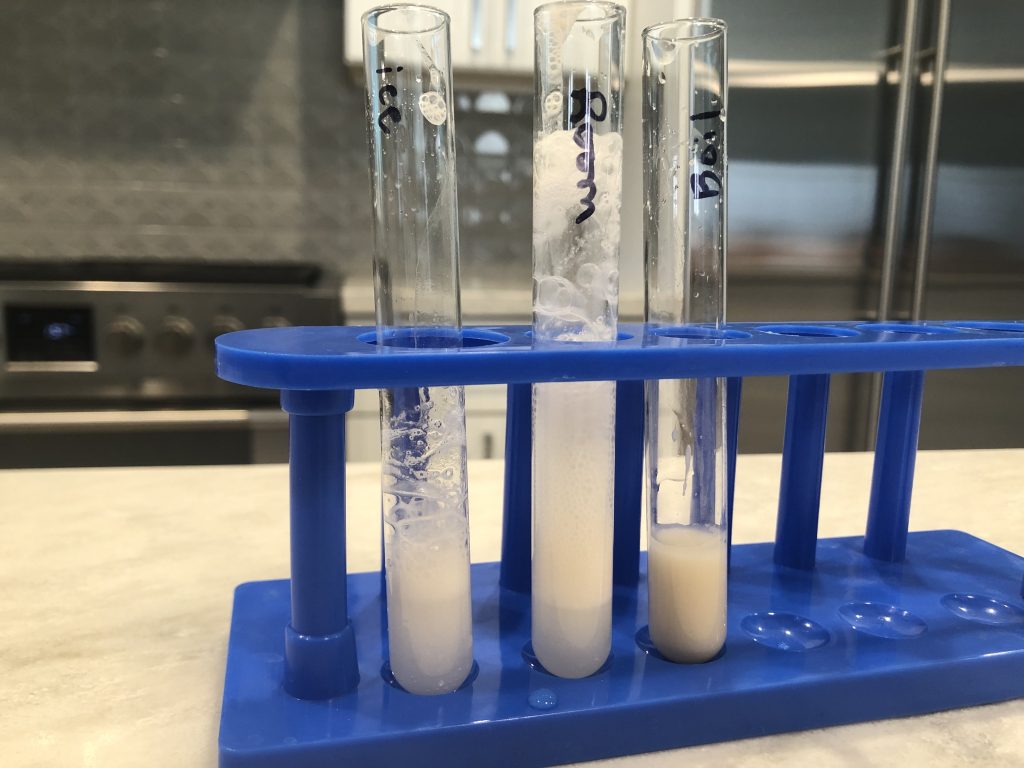
effect of temperature on catalase activity experiment
You will use room temperature catalase, ice cold catalase, and boiled catalase.
1. You have already prepared your ice cold enzyme by preparing an ice bath and placing the test tube labeled ice containing 2 ml of catalase in the ice bath.
2. Use the permanent marker to label one test tube room. Using the pipette in the enzyme add 2 ml of enzyme to the room tube; this will be your room temperature enzyme.
3. Take all of the enzyme solution you have left and either boil it for a few minutes on the stove or microwave it in a microwave safe container. Dispose of the plastic cup labeled enzyme and the transfer pipette in the enzyme cup in the trash. Let the boiled enzyme cool to room temperature.
4. Use the permanent marker to label a plastic cup from the lab kit boiled enzyme. Pour the room temperature boiled enzyme into the cup.
5. Use the permanent marker to label one test tube boiled. Using a new sterile, transfer pipette add 2 ml of the boiled enzyme to the boiled tube; this will be your boiled enzyme.
6. Using the pipette in the H2O2 add 2 ml of H2O2 to all 3 test tubes.
7. Observe the bubbles (oxygen) produced in each test tube. Use the scale of 0-3 to describe the rate of reaction and record your results in the data table in the Enzymes Question Document.
0=no reaction (no bubbles formed)
1 = few bubbles formed
2 = some bubbles formed
3 = many bubbles formed
8.  Take a photo with your photo ID of the test tubes in the test tube rack.
Take a photo with your photo ID of the test tubes in the test tube rack.
9. Clean the test tube thoroughly with the test tube brush and soap and water. Remove the ice, room, and boiled labels from the test tubes using paper towel and isopropyl alcohol.
10. Pour the liquid in the plastic cups down the sink. Run water for at least 30 seconds. Dispose of the cups and transfer pipettes in the trash.
11. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash. Dispose of left over surface disinfectant by pouring it down the sink and running water for at least 30 seconds.
12. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.