OSMOSIS
LEARNING OBJECTIVES
Compare and contrast diffusion and osmosis.
Describe what is meant by a selectively permeable membrane.
Identify the effect of isotonic, hypotonic, an hypertonic solutions on a cell.
MCCCD COURSE COMPETENCIES
Describe and utilize the process of scientific inquiry, its realm, and limitations.
Explain and demonstrate the mechanisms of microbial growth and metabolism.
Name foods that owe their existence to microbes and describe food spoilage, food preservation, and prevention of food-borne diseases.
Describe physical, chemical, and antibiotic methods of microbial control.
PHOTO REQUIREMENTS
Take a photo with your photo ID during the lab exercises when you see this icon.

Paste the photos in the Osmosis Questions Document.
INTRODUCTION
Cell membranes are also known as plasma membranes or cytoplasmic membranes. Cell membranes are a cell basic, prokaryotic and eukaryotic cells have a cell membrane. The cell membrane separates the cell from the environment. In prokaryotes the cell membrane lies beneath the cell wall.
Functions of the cell membrane include acting as a boundary and controlling traffic in and out of the cell (membrane transport). Components of the prokaryotic cell membrane include phospholipids (40%) and proteins (60%). The phospholipids are arranged in a bilayer with the tails of the phospholipids facing each other. Phospholipids have hydrophilic (water-loving) heads and hydrophobic (water-fearing) tails. The tails wiggle and add to the fluidity of the cell membrane; cell membranes are about as fluid as olive oil. The phospholipid bilayer allows some molecules to easily pass across the membrane and does not allow other molecules to easily pass across the membrane. Cell membranes are therefore selectively permeable. Most of the phospholipid bilayer is made up of the hydrophobic core. Hydrophobic molecules are allowed easy passage across the membrane. Hydrophilic molecules cannot pass the hydrophobic core so they cannot easily pass across the membrane. The proteins in a membrane can act as transport channels. These transport channels can be used to transport specific molecules across the membrane. Proteins in the membrane can also be receptors, carriers, or recognition proteins.
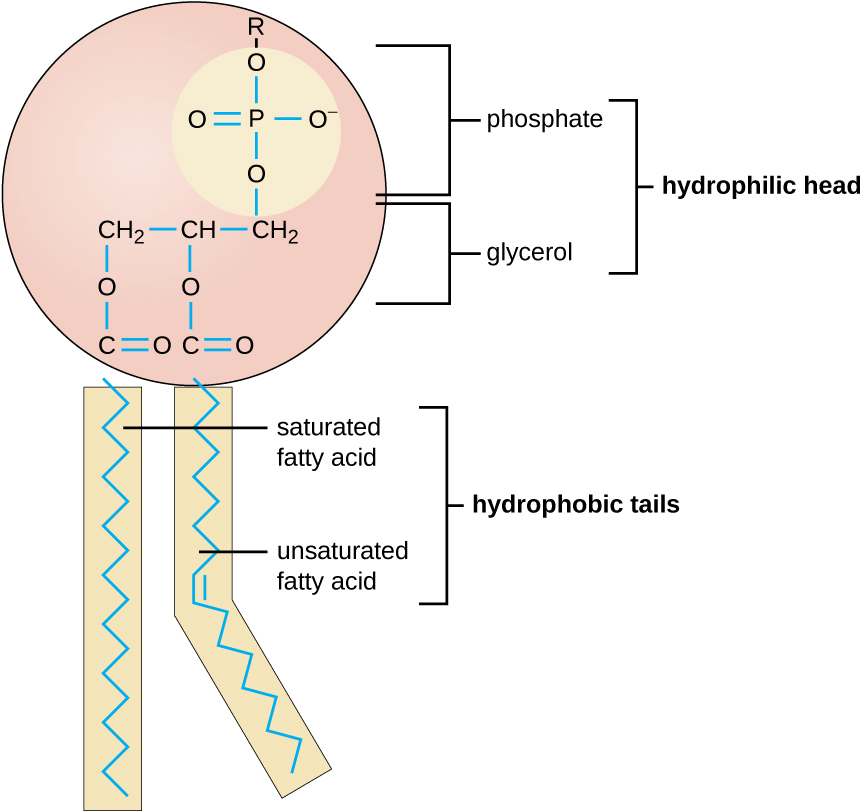
Image from openstax Microbiology
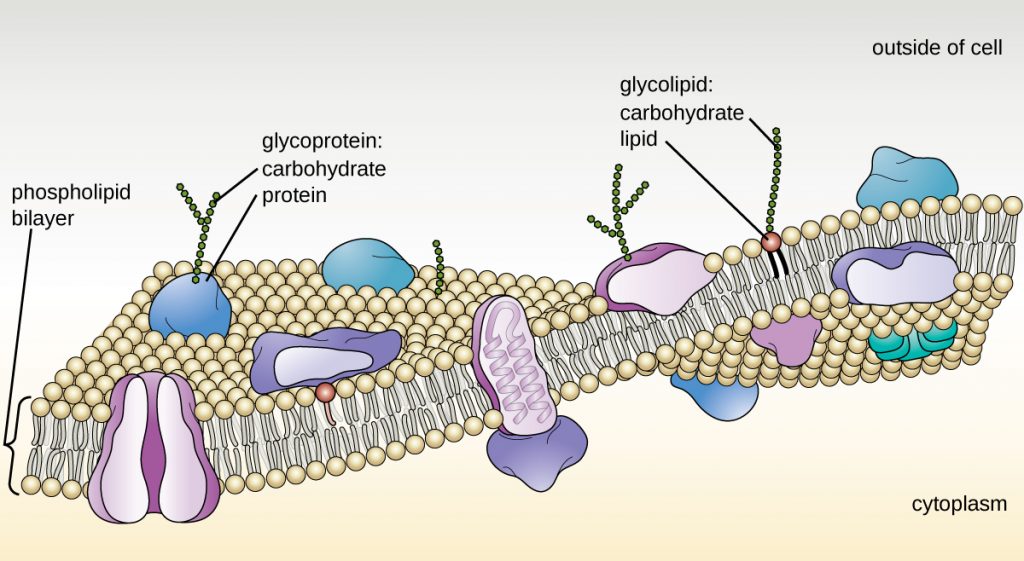
Image from openstax Microbiology
Membrane transport is defined as passage of a substance across a membrane. Remember the cell membrane is selectively permeable. There are two types of membrane transport; active transport and passive transport. Passive transport is movement of a substance across a membrane from an area of high concentration to an area of low concentration. There is no energy or ATP required. The goal of passive transport is equilibrium across the membrane. There are three types of passive transport; diffusion, facilitated diffusion, and osmosis.
There are three types of passive transport; diffusion, facilitated diffusion, and osmosis.
diffusion
Diffusion is movement of a substance from an area of high concentration to an area of low concentration across a membrane. No energy or ATP is required and the goal of diffusion is equilibrium across the membrane. Oxygen and carbon dioxide use diffusion to cross a membrane. Facilitated diffusion is movement of a substance from an area of high concentration to an area of low concentration across a membrane using a protein transport channel. The protein transport channel can be specific or non-specific. No energy or ATP is required and the goal of facilitated diffusion is equilibrium across the membrane. Glucose and fructose use facilitated diffusion to cross a membrane.
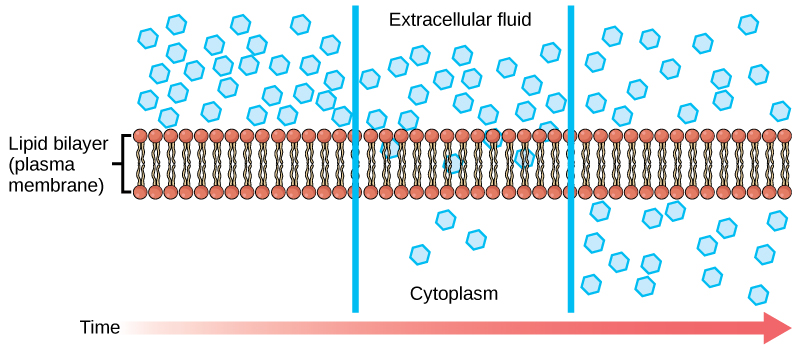
Image from openstax Biology 2e
osmosis
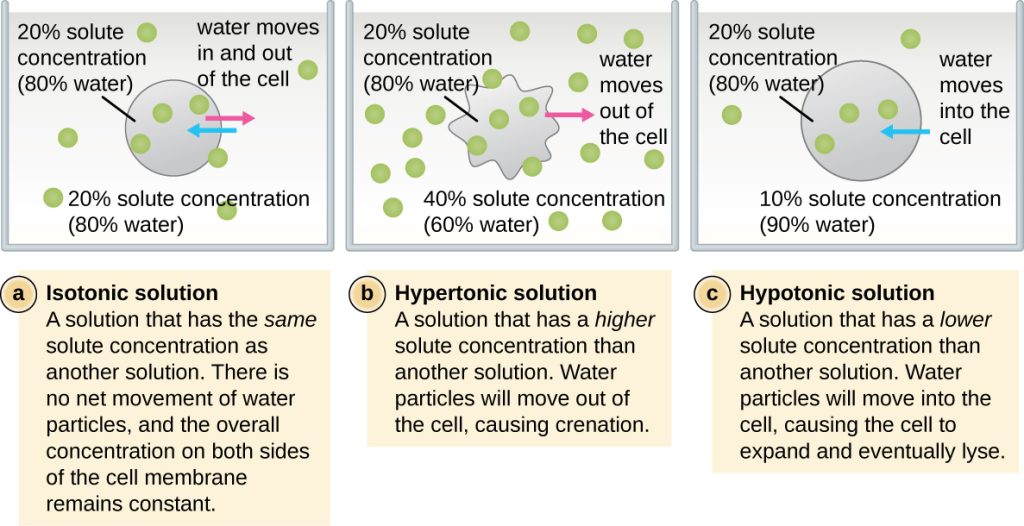
Osmosis is movement of water from an area of high water concentration to an area of low water concentration. There is no energy or ATP required. The goal of osmosis is equilibrium across the membrane.
If there is not equilibrium across the membrane in what direction will water osmose? That depends on the solution the cell is in. An isotonic solution has the same water concentration inside and outside the cell. Equilibrium has already been achieved, no osmosis is required. A hypertonic solution has a higher solute concentration outside the cell. Water will osmose out of the cell to achieve equilibrium and the cell will shrivel. This is why jelly and pickles last so long in the refrigerator; they are hypertonic solutions of sugar (jelly) or salt (pickles). A hypotonic solution has a lower solute concentration outside the cell. Water will osmose into the cell to achieve equilibrium and the cell will lyse.
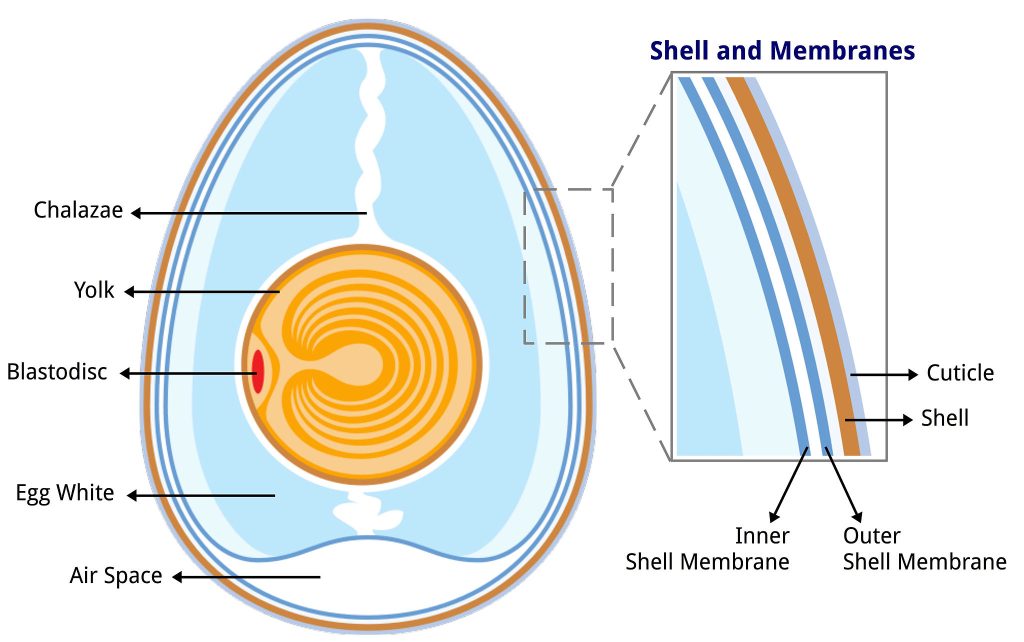
A chicken egg has a cuticle and shell that protect an underlying membrane. The cuticle keeps bacteria and dust out of the egg. In the U.S., Japan, Australia, New Zealand, and parts of Scandinavia, eggs are refrigerated because eggs are washed extensively which removes the cuticle. Without a cuticle, eggs need to be kept cold—not for the product itself, but to discourage bacterial growth in and on the egg. In most European and Asian countries eggs are not refrigerated because eggs are not washed extensively leaving the cuticle intact so the eggs can be stored at room temperature. The eggshell is made almost entirely of calcium carbonate. We will soak the egg in vinegar (4-5% acetic acid). The acetic acid breaks apart the calcium and carbonate dissolving the shell releasing carbon dioxide gas seen as bubbles on the shell. The chicken egg membrane is a selectively permeable membrane. After the shell is removed, we will expose the egg to various liquids and determine if osmosis occurred.
SCIENTIFIC METHOD
Question-Does osmosis occur? If osmosis does occur, in what direction does water osmose?
Hypothesis-Water will osmose from an area of high water concentration to an area of low water concentration.
Experimentation-You will soak a raw egg in various liquids. You will measure the amount of liquid remaining in the cup. You will keep an egg in the refrigerator to use as a comparison.
osmosis procedure
| REQUIRED SUPPLIES FROM THE LAB KIT |
| 100 ml Graduated Cylinder
|
| Plastic Cups (3)
|
| Test Tube Brush
|
| REQUIRED SUPPLIES YOU PROVIDE |
| Raw Eggs 2 (keep 1 egg in the refrigerator for the duration of the lab to use as a comparison) |
White distilled vinegar (4-5% acetic acid, usually specified on the label)  |
| Water (bottled/reverse osmosis or let tap water sit out for 24 hours) USED AFTER YOU SOAK THE EGG IN VINEGAR FOR 48 HOURS AND SUGAR SOLUTION FOR 24 HOURS |
| White sugar (13 Tablespoons) |
| Aluminum foil or plastic wrap |
| Spoon |
| Paper towels |
| Cup or bowl for surface disinfectant |
| Bleach |
| Dish soap |
| Permanent marker |
OSMOSIS PART ONE – PLACE THE EGG IN DISTILLED WHITE VINEGAR
A chicken egg contains 76% water. Distilled white vinegar contains 96% water and 4-5% acetic acid.
1. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.
2. Prepare surface disinfectant. Using the 100 ml graduated cylinder add 180 ml of tap water to a cup or bowl. Using the 100 ml graduated cylinder now add 20 ml of bleach to the cup or bowl. The surface disinfectant (10% bleach solution) is now ready to use.
3. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash.
4. Use the permanent marker to label a plastic cup from the lab kit vinegar.
5. Clean the 100 ml graduated cylinder with the test brush and soap and water.
6. Use the 100 ml graduated cylinder to measure 200 ml of white distilled vinegar and pour vinegar into the cup.
7. Use a large spoon gently add the egg into the vinegar. Cover the cup with aluminum foil or plastic wrap.
8. Let the egg sit in the vinegar for 48 hours at room temperature. Clean the 100 ml graduated cylinder thoroughly with soap and water and the test tube brush.
9. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash. Dispose of left over surface disinfectant by pouring it down the sink and running water for at least 30 seconds.
10. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.
AFTER 48 HOUR INCUBATION
1. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.
2. Prepare surface disinfectant. Using the 100 ml graduated cylinder add 180 ml of tap water to a cup or bowl. Using the 100 ml graduated cylinder now add 20 ml of bleach to the cup or bowl. The surface disinfectant (10% bleach solution) is now ready to use.
3. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash.
4. Use a large spoon carefully remove the egg from the vinegar cup and place the egg on a piece of paper towel. Place the egg from the refrigerator next to the egg you removed from the vinegar cup.
5.  Take a photo with your photo ID of the eggs. Do not pour out the vinegar in the cup! Do not throw away the egg!
Take a photo with your photo ID of the eggs. Do not pour out the vinegar in the cup! Do not throw away the egg!
6. Use the 100 ml graduated cylinder to measure the amount of vinegar left in the cup. Record your result in the data table in the Osmosis Questions document. Pour the vinegar down the sink and let the water run for 30 seconds. Clean the 100 ml graduated cylinder thoroughly with soap and water and the test tube brush. Place the comparison egg back in the refrigerator. Dispose of the cup in the trash.
OSMOSIS PART TWO – PLACE THE EGG IN A 70% SUGAR SOLUTION
A chicken egg contains 76% water. The sugar solution is 30% water and 70% sugar.
1. Clean the 100 ml graduated cylinder with the test brush and soap and water.
2. Using the graduated cylinder, add 100 ml of water (tap is fine) to a pot. Add 13 Tablespoons of white sugar to the pot.
3. Place the pot with the sugar solution on the stove over medium heat. Stir constantly. Allow the sugar solution to come to a boil and continue to boil it until it is clear. Remove the pot from the heat. Let the sugar solution cool to room temperature.
4. Use the permanent marker to label a plastic cup from the lab kit sugar solution.
5. Clean the 100 ml graduated cylinder with the test brush and soap and water. Using the graduated cylinder, add 200 ml of the sugar solution to the cup. If you have less than 200 ml of sugar solution, bring the amount up to 200 ml with water. If you have more than 200 ml of sugar solution, only use 200 ml.
6. Using a clean, large, spoon gently add the egg that was soaked in vinegar to the sugar solution. Cover the cup with aluminum foil or plastic wrap.
7. Let the egg sit in sugar solution for 24 hours at room temperature. Clean the 100 ml graduated cylinder thoroughly with soap and water and the test tube brush.
8. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash. Dispose of left over surface disinfectant by pouring it down the sink and running water for at least 30 seconds.
9. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.
AFTER 24 HOUR INCUBATION
1. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.
2. Prepare surface disinfectant. Using the 100 ml graduated cylinder add 180 ml of tap water to a cup or bowl. Using the 100 ml graduated cylinder now add 20 ml of bleach to the cup or bowl. The surface disinfectant (10% bleach solution) is now ready to use.
3. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash.
4. Use a large spoon carefully remove the egg from the sugar solution cup and place the egg on a piece of paper towel. Place the egg from the refrigerator next to the egg you removed from the sugar solution cup.
5.  Take a photo with your photo ID of the eggs. Do not pour out the sugar solution in the cup! Do not throw away the egg!
Take a photo with your photo ID of the eggs. Do not pour out the sugar solution in the cup! Do not throw away the egg!
6. Use the 100 ml graduated cylinder to measure the amount of sugar solution left in the cup. Record your result in the data table in the Osmosis Questions document. Pour the sugar solution down the sink and let the water run for 30 seconds. Clean the 100 ml graduated cylinder thoroughly with soap and water and the test tube brush. Place the comparison egg back in the refrigerator. Dispose of the cup in the trash.
OSMOSIS PART THREE – PLACE THE EGG IN WATER
A chicken egg contains 76% water. Water is 100% water.
1. Use the permanent marker to label a plastic cup from the lab kit water.
2. Use the 100 ml graduated cylinder to measure 200 ml of water (bottled/reverse osmosis or let tap water sit out for 24 hours) and pour the water into the cup.
3. Using a clean, large, spoon gently add the egg into the water. Cover the cup with aluminum foil or plastic wrap.
4. Let the egg sit in water for 24 hours at room temperature. Clean the 100 ml graduated cylinder with the brush and soap and water.
5. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash. Dispose of left over surface disinfectant by pouring it down the sink and running water for at least 30 seconds.
6. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.
AFTER 24 HOUR INCUBATION
1. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.
2. Prepare surface disinfectant. Using the 100 ml graduated cylinder add 180 ml of tap water to a cup or bowl. Using the 100 ml graduated cylinder now add 20 ml of bleach to the cup or bowl. The surface disinfectant (10% bleach solution) is now ready to use.
3. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash.
4. Use a large spoon carefully remove the egg from the water cup and place the egg on a piece of paper towel. Place the egg from the refrigerator next to the egg you removed from the water cup.
5.  Take a photo with your photo ID of the eggs. Do not pour out the water in the cup!
Take a photo with your photo ID of the eggs. Do not pour out the water in the cup!
6. Use the 100 ml graduated cylinder to measure the amount of water left in the cup. Record your result in the data table in the Osmosis Questions document. Pour the water down the sink. Clean the 100 ml graduated cylinder thoroughly with soap and water and the test tube brush.
7. Dispose the egg and cup in the trash.
8. Disinfect your work surface with the surface disinfectant by applying surface disinfectant with a paper towel, allowing it to remain damp for 2 minutes, and then wiping away any remaining disinfectant with a dry paper towel. Throw the used paper towels in the trash. Dispose of left over surface disinfectant by pouring it down the sink and running water for at least 30 seconds.
9. Wash your hands thoroughly with soap and water. Dry your hands with paper towel.