7 Gene Regulation in Bacteria: The Operon Model
Learning Objectives
Upon completing this chapter, you will be able to:
- Explain why gene regulation is essential for bacterial survival and efficiency.
- Differentiate between structural genes and regulatory genes.
- Describe the structure of a bacterial operon, including the roles of the promoter, operator, and structural genes.
- Define and explain the roles of repressors, activators, and inducers in gene regulation.
- Distinguish between constitutively expressed genes and regulated genes.
- Compare and contrast inducible operons and repressible operons, providing examples of each.
- Explain the mechanism of regulation for the lactose (lac) operon in E. coli, including the roles of the repressor protein and lactose as an inducer.
- Explain the mechanism of regulation for the tryptophan (trp) operon in E. coli, including the roles of the repressor protein and tryptophan as a co-repressor.
Introduction to Gene Regulation in Bacteria
All cells must manage their resources efficiently. Genomic DNA contains the blueprints for all cellular components, divided into structural genes, which encode products like enzymes and cellular structures, and regulatory genes, which encode products that control the expression of other genes. Gene expression, the process of turning on a gene to produce RNA and protein, is a highly regulated process. In single-celled organisms like prokaryotes, gene regulation primarily ensures that the cell’s energy and resources are not wasted making proteins that are not needed at that particular time. This allows bacteria to adapt quickly to changes in their environment.
Understanding the mechanisms that control gene expression is fundamental. Malfunctions in this process in humans can lead to diseases like cancer. In the context of infectious diseases, understanding the interplay between a pathogen’s gene expression and its human host is crucial. Gene regulation involves a complex network of interactions within a cell, responding to environmental signals through signaling molecules and DNA itself, ultimately leading to the expression of some genes and the suppression of others. While prokaryotes and eukaryotes share some regulatory mechanisms, prokaryotic gene regulation is generally less complex, primarily occurring at the level of transcription.
The Operon Model: Coordinated Gene Control
In bacteria and archaea, genes encoding proteins with related functions (e.g., enzymes in a specific metabolic pathway) are often organized together in a block within the genome called an operon. These genes are transcribed together as a single unit from one promoter, resulting in a polycistronic transcript (a single mRNA molecule that codes for multiple proteins). This coordinated control ensures that all proteins needed for a particular pathway are synthesized simultaneously when required, or not at all if they are not needed.
For example, in E. coli, all structural genes encoding enzymes necessary to utilize the sugar lactose as an energy source are grouped in the lactose (or lac) operon, under the control of a single promoter. The pioneering work of French scientists François Jacob and Jacques Monod on the lac operon first revealed this organizational structure, earning them the Nobel Prize in 1965. Prokaryotic operons serve as excellent models for understanding gene regulation more broadly.
Key Components of an Operon:
Each operon includes DNA sequences within a regulatory region that influence its own transcription. This region typically includes:
- Promoter: The DNA sequence where RNA polymerase binds to initiate transcription.
- Operator: A DNA sequence located between the promoter and the transcriptional start site of the first structural gene. Regulatory proteins bind here.
Regulatory Molecules:
- Transcription Factors: Proteins encoded by regulatory genes that bind to DNA sequences (like the operator or other sites near the promoter) to influence transcription.
- Repressor: A transcription factor that suppresses or turns off gene transcription. It typically binds to the operator, physically blocking RNA polymerase from transcribing the structural genes.
- Activator: A transcription factor that increases or turns on gene transcription, often by helping RNA polymerase bind more effectively to the promoter.
- Inducer: A small molecule that interacts with a repressor or an activator to modulate its activity, thereby either activating or repressing transcription.
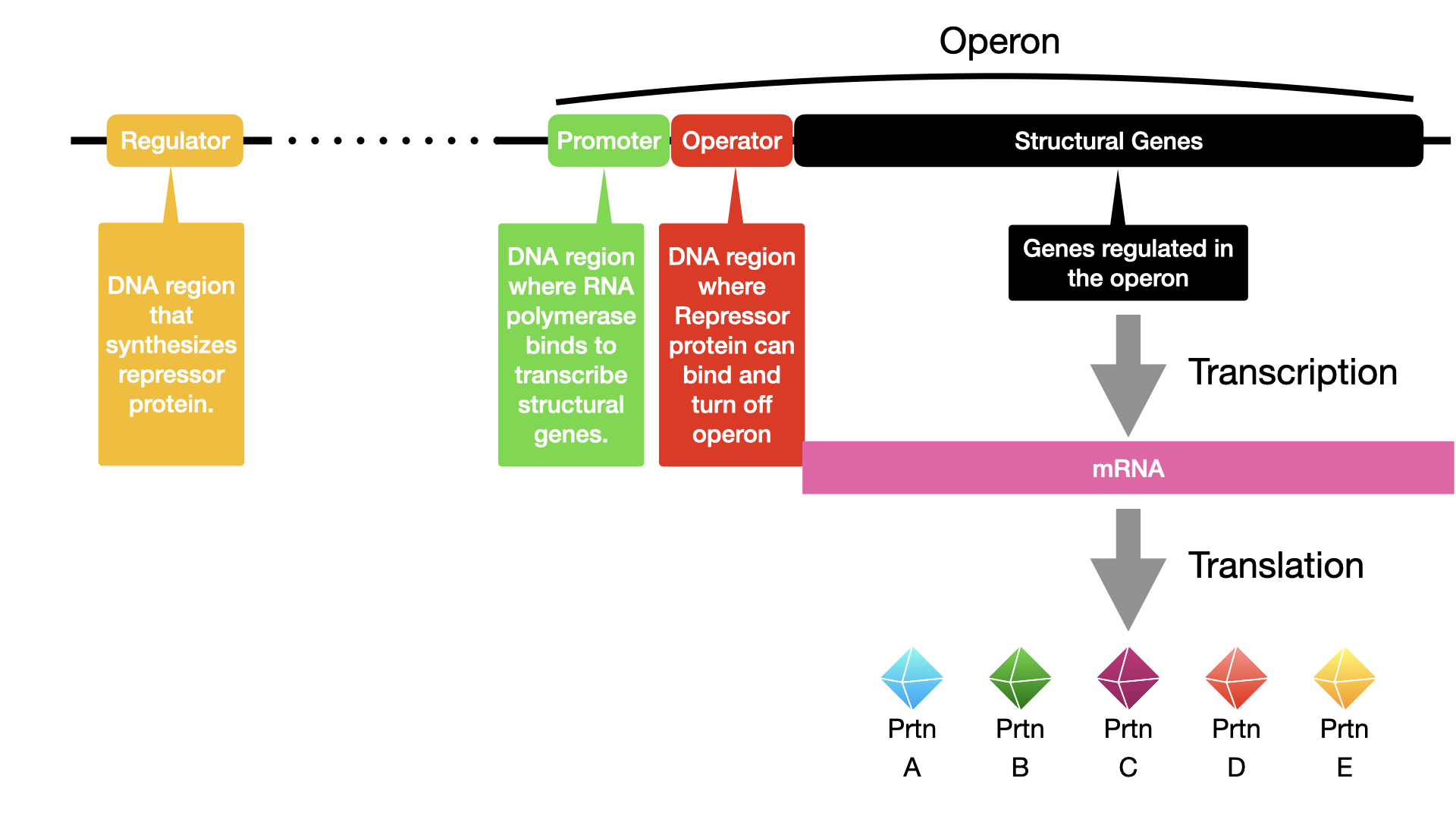
Not all operons are regulated. Some, whose gene products are consistently required for basic cellular functions (housekeeping genes involved in DNA replication, repair, core metabolism), are constitutively expressed. This means they are transcribed and translated continuously. However, many prokaryotic operons are expressed only when needed and are tightly regulated.
Types of Regulated Operons: Inducible vs. Repressible
Regulated operons in prokaryotes are commonly controlled by repressors binding to operator regions. They can be classified into two main types based on their default state and how they respond to external stimuli:
- Inducible Operons:
- Default state: OFF. The genes are not actively transcribed.
- Activation: Transcription is turned ON or induced in response to a specific molecule, usually the substrate the operon’s enzymes will act upon. The molecule that triggers this is an inducer.
- Function: Often encode enzymes involved in catabolic pathways (breaking down a substance). Example: The lac operon.
- Repressible Operons:
- Default state: ON. The genes are actively transcribed.
- Repression: Transcription is turned OFF or repressed when a specific molecule, often the end product of the operon’s biosynthetic pathway, accumulates. This molecule often acts as a co-repressor.
- Function: Typically encode enzymes required for anabolic pathways (synthesizing a substance). Example: The trp operon.
The Lactose (lac) Operon: An Inducible System
The lactose (lac) operon in E. coli is a classic example of an inducible operon. It contains three structural genes (lacZ, lacY, and lacA) that encode proteins necessary to acquire and process the disaccharide sugar lactose from the environment, breaking it down into glucose and galactose for energy. Since these proteins are only needed when lactose is present, the operon is regulated to prevent wasteful enzyme production.
Key Components of the lac Operon:
- Regulator gene (lacI): Located elsewhere on the chromosome, this gene is constitutively expressed and produces the Lac repressor protein.
- Promoter (P): The binding site for RNA polymerase.
- Operator (O): The binding site for the Lac repressor protein. It overlaps with the promoter.
- Structural Genes: lacZ, lacY, lacA.
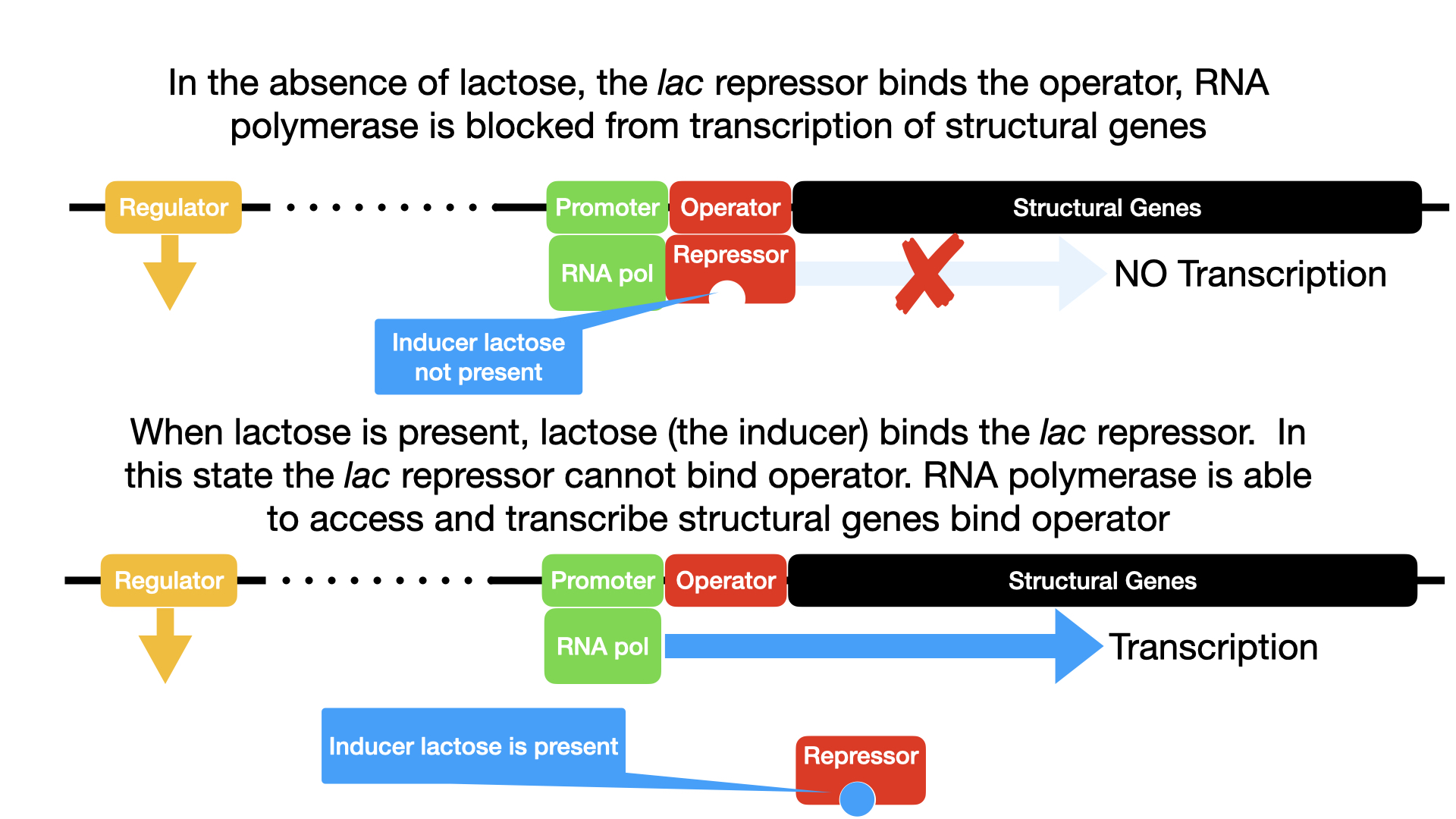
Mechanism of Regulation:
- When Lactose is ABSENT (Operon OFF):
- The Lac repressor protein is continuously synthesized.
- In the absence of lactose, the Lac repressor protein is in an active shape and binds tightly to the operator sequence of the lac operon.
- The binding of the repressor to the operator physically blocks RNA polymerase (which can still bind to the promoter) from moving forward and transcribing the structural genes.
- Therefore, no mRNA is made from the structural genes, and consequently, the enzymes for lactose metabolism are not produced. The operon is OFF.
- When Lactose is PRESENT (Operon ON – Induced):
- If lactose becomes available to the cell, some of it is converted into allolactose (an isomer of lactose).
- Allolactose acts as the inducer. It binds to a specific site on the Lac repressor protein.
- This binding causes a conformational change (change in shape) in the repressor protein, making it unable to bind to the operator DNA.
- With the repressor no longer bound to the operator, the operator site is free.
- RNA polymerase can now move past the operator and transcribe the structural genes (lacZ, lacY, lacA) into a single polycistronic mRNA.
- This mRNA is then translated into the three enzymes required for lactose metabolism. The operon is ON.
- These enzymes then break down lactose. As lactose (and therefore allolactose) concentrations decrease due to metabolism, the inducer molecules detach from the repressor. The repressor then returns to its original active shape, re-binds to the operator, and turns the operon OFF again, thus ensuring enzymes are only made when their substrate is present.
Note: The lac operon is also subject to another layer of control called catabolite repression, which involves glucose levels and an activator protein (CAP). This ensures that E. coli preferentially uses glucose when available, even if lactose is also present. This is a more advanced level of control not detailed in the provided video.
The Tryptophan (trp) Operon: A Repressible System tryptophan
The tryptophan (trp) operon in E. coli is a prime example of a repressible operon. It contains five structural genes (trpE, trpD, trpC, trpB, trpA) that encode enzymes necessary for the cell to synthesize the amino acid tryptophan. Tryptophan is an essential building block for proteins, so cells need a way to make it if it’s not available from the environment, but also to stop making it if it’s already plentiful, to conserve energy.
Key Components of the trp Operon:
- Regulator gene (trpR): Located elsewhere, this gene constitutively produces the Trp repressor protein, but in an inactive form (an aporepressor).
- Promoter (P): The binding site for RNA polymerase.
- Operator (O): The binding site for the active Trp repressor-co-repressor complex.
- Structural Genes: trpE, trpD, trpC, trpB, trpA.
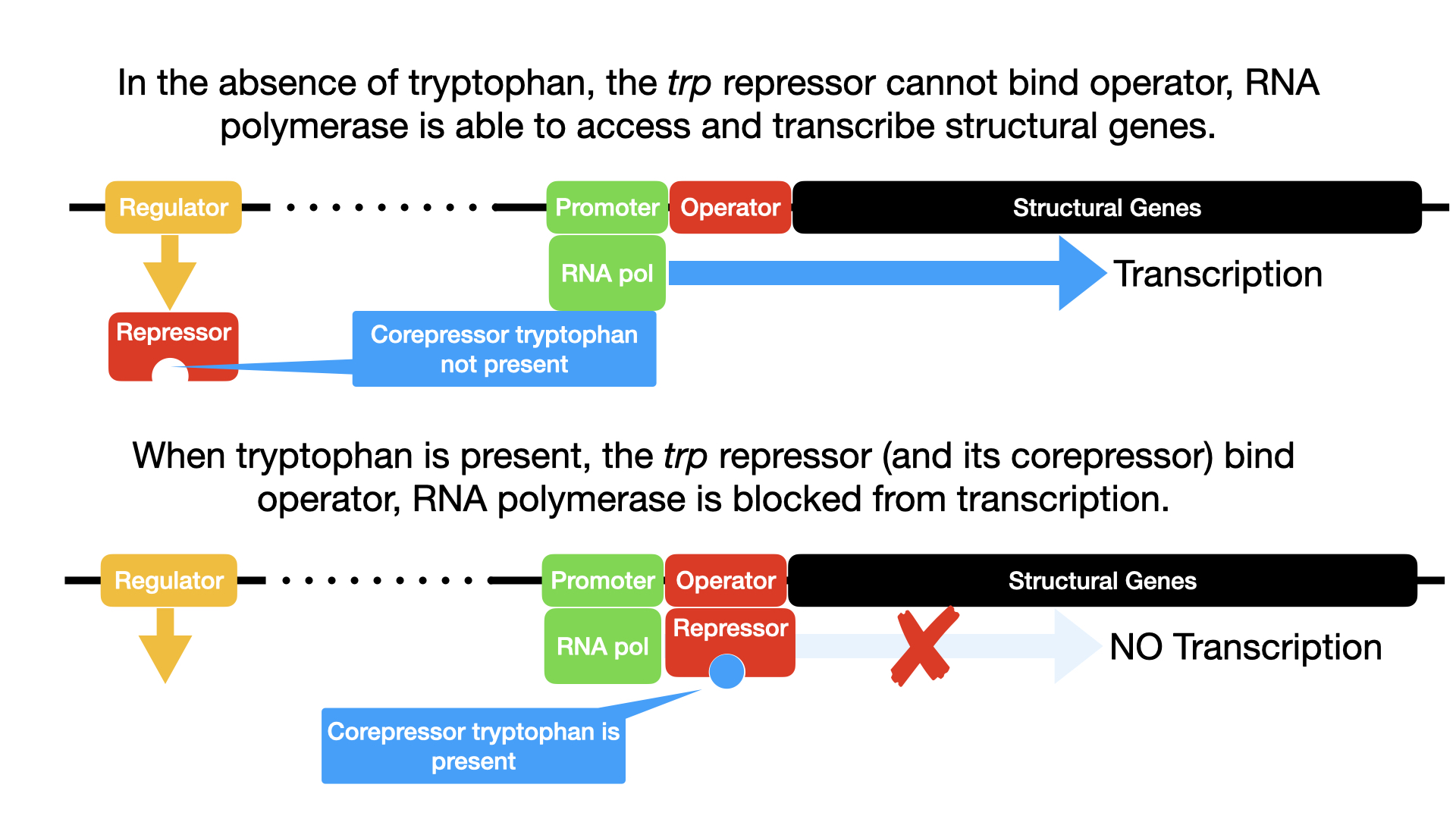
Mechanism of Regulation:
- When Tryptophan is LOW (Operon ON):
- The Trp repressor protein is synthesized in an inactive form that, by itself, cannot bind to the operator.
- The operator site is therefore unoccupied by the repressor.
- RNA polymerase can bind to the promoter and freely transcribe the five structural genes into a polycistronic mRNA.
- This mRNA is translated into the enzymes that collectively synthesize tryptophan.
- The operon is ON, and the cell produces tryptophan because it needs it.
- When Tryptophan is HIGH (Operon OFF – Repressed):
- If tryptophan becomes abundant in the cell (either from the environment or from the cell’s own synthesis), it acts as a co-repressor.
- Tryptophan molecules bind to the inactive Trp repressor protein.
- This binding of tryptophan (the co-repressor) to the repressor protein causes a conformational change in the repressor, activating it.
- The now active repressor-co-repressor complex is able to bind tightly to the operator sequence of the trp operon.
- The binding of this complex to the operator physically blocks RNA polymerase from accessing and transcribing the structural genes.
- Transcription stops, and no more enzymes for tryptophan synthesis are produced, as the cell already has sufficient tryptophan. The operon is OFF.
- As the cell uses up its tryptophan supply (e.g., for protein synthesis), the tryptophan concentration decreases. Tryptophan molecules will then dissociate from the repressor. The repressor reverts to its inactive form, detaches from the operator, and RNA polymerase can once again transcribe the operon, turning it back ON.
Chapter Summary
Gene regulation in bacteria is vital for their adaptation and efficient use of resources, primarily occurring at the level of transcription. The operon model describes how bacteria coordinately regulate genes with related functions. An operon consists of a promoter, an operator, and structural genes transcribed as a polycistronic mRNA. Regulatory proteins like repressors (which block transcription) and activators (which enhance it), often influenced by small molecules like inducers or co-repressors, control operon expression.
- Inducible operons, like the lactose (lac) operon, are normally OFF. The lac operon’s repressor binds the operator, preventing transcription. When lactose (converted to allolactose, the inducer) is present, it binds to the repressor, causing it to detach from the operator, allowing transcription of genes for lactose metabolism.
- Repressible operons, like the tryptophan (trp) operon, are normally ON. The trp operon’s repressor is initially inactive. When tryptophan (the co-repressor) levels are high, it binds to and activates the repressor. This active complex then binds to the operator, blocking transcription of genes for tryptophan synthesis.
These sophisticated regulatory mechanisms allow bacteria to precisely control gene expression in response to their metabolic needs and environmental conditions.
Media Attributions
- Operon general.001
- Lactose operon figure.001
- Trp operon figure ver 2.001

