6 Viral Replication
Learning Objectives
Upon completing this chapter, you will be able to:
- Explain why viruses are dependent on host cells for replication.
- Describe the key differences between virulent and temperate bacteriophages.
- Outline and describe the five stages of the lytic cycle of bacteriophage replication.
- Outline and describe the stages of the lysogenic cycle of bacteriophage replication, including integration, prophage formation, and induction.
- Define key terms such as prophage, lysogen, lysogeny, and lysogenic conversion.
- Explain how lysogenic conversion can alter the phenotype of a host bacterium.
Introduction to Viral Replication
All viruses are obligate intracellular parasites, meaning they absolutely depend on living host cells for their reproduction and metabolic processes. By themselves, viruses do not encode all the enzymes necessary for their own replication. However, once inside a host cell, a virus can effectively commandeer the cellular machinery to produce many copies of itself, forming new viral particles.
The location of viral replication varies depending on the virus and the host cell type:
- Bacteriophages (viruses that infect bacteria) replicate only in the cytoplasm, as prokaryotic cells lack a nucleus and other membrane-bound organelles.
- In eukaryotic cells:
- Most DNA viruses replicate inside the nucleus, utilizing host cell machinery for DNA replication and transcription. An exception includes large DNA viruses like poxviruses, which can replicate in the cytoplasm.
- Most RNA viruses that infect animal cells replicate in the cytoplasm. An important exception, like the Influenza virus, has replication steps in both the nucleus and cytoplasm.
The Life Cycle of Bacteriophages (Viruses with Prokaryote Hosts)
The life cycle of bacteriophages has served as an excellent model for understanding how viruses interact with and affect the cells they infect. Many of the fundamental processes observed in bacteriophage replication are also seen in the replication of eukaryotic viruses. These interactions can lead to various outcomes, including the immediate death of the host cell or the establishment of a latent or chronic infection.
Bacteriophages can be broadly categorized based on their replication strategy:
- Virulent phages typically lead to the death of the host cell through cell lysis. They exclusively follow the lytic cycle.
- Temperate phages, on the other hand, can choose between two paths after infecting a host cell: they can either enter the lytic cycle or they can integrate their genome into the host chromosome, becoming part of the host. This latter state is part of the lysogenic cycle. The integrated phage genome can remain dormant until it is induced to produce newly assembled viruses (progeny viruses).
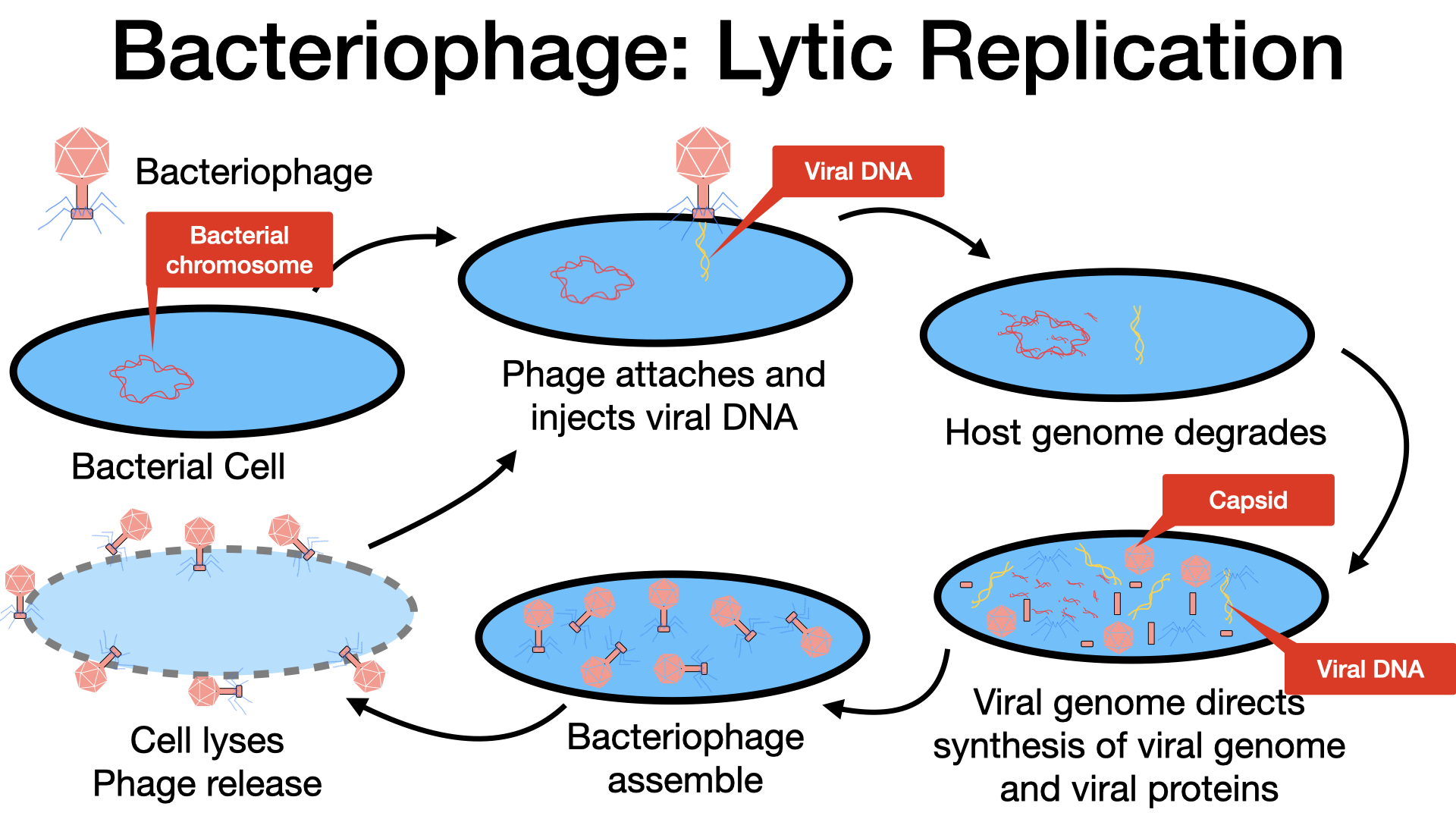
The Lytic Cycle: Viral Overthrow and Host Cell Destruction
During the lytic cycle, a virulent bacteriophage takes control of the host cell, directs the production of new phage components, assembles new phages, and ultimately destroys the host cell to release the progeny. The T-even phage (e.g., T4 phage) is a well-studied example of a virulent phage that undergoes this cycle. The lytic cycle can be broken down into five distinct stages:
-
Attachment (Adsorption): This is the initial stage where the phage specifically binds to the surface of a bacterial host cell. The phage interacts with specific bacterial surface receptors, such as lipopolysaccharides or particular outer membrane proteins. This interaction is highly specific, meaning most phages have a narrow host range, often infecting only one species of bacteria or even specific strains within a species. This specificity is crucial for phage therapy and phage typing.
-
Penetration (Entry): Following attachment, the phage injects its viral genome into the host cell. This is often achieved through a contraction of the phage’s tail sheath, which acts like a hypodermic needle, pushing the tail core through the bacterial cell wall and membrane. The phage head (capsid) and other remaining components typically stay outside the bacterium.
-
Biosynthesis (Synthesis): Once the viral DNA has entered the host cell, the virus begins to synthesize new viral components. A critical early step often involves the virus producing endonucleases (enzymes) that degrade the host bacterium’s chromosome. This effectively shreds the bacterial “instruction manual,” leaving the viral genome as the primary template for the cell’s machinery. The phage then hijacks the host cell’s ribosomes, enzymes, and metabolic pathways to:
- Replicate its viral genome multiple times.
- Transcribe viral genes into messenger RNA (mRNA).
- Translate the viral mRNA into viral proteins, such as capsomeres (capsid proteins), tail sheath proteins, base plates, tail fibers, and viral enzymes (like those needed for DNA replication or later, for cell lysis). Typically, genes for viral polymerases are expressed early, while genes for structural proteins like capsid and tail components are expressed later.
-
Maturation (Assembly): During this phase, the newly synthesized viral components (genomes and proteins) self-assemble into complete, new viral particles or virions. This assembly is a complex, often spontaneous process, but can also be guided by specific viral proteins. Many new bacteriophages are built within the confines of the host cell.
-
Release (Lysis): This is the final stage where the newly assembled mature viruses burst out of the host cell. The bacterial cell wall is disrupted by phage-encoded proteins. This process, called lysis, results in the death of the host cell and the liberation of progeny viruses into the environment, ready to infect new susceptible host cells and repeat the cycle.
The Lysogenic Cycle: Viral Integration and Latency
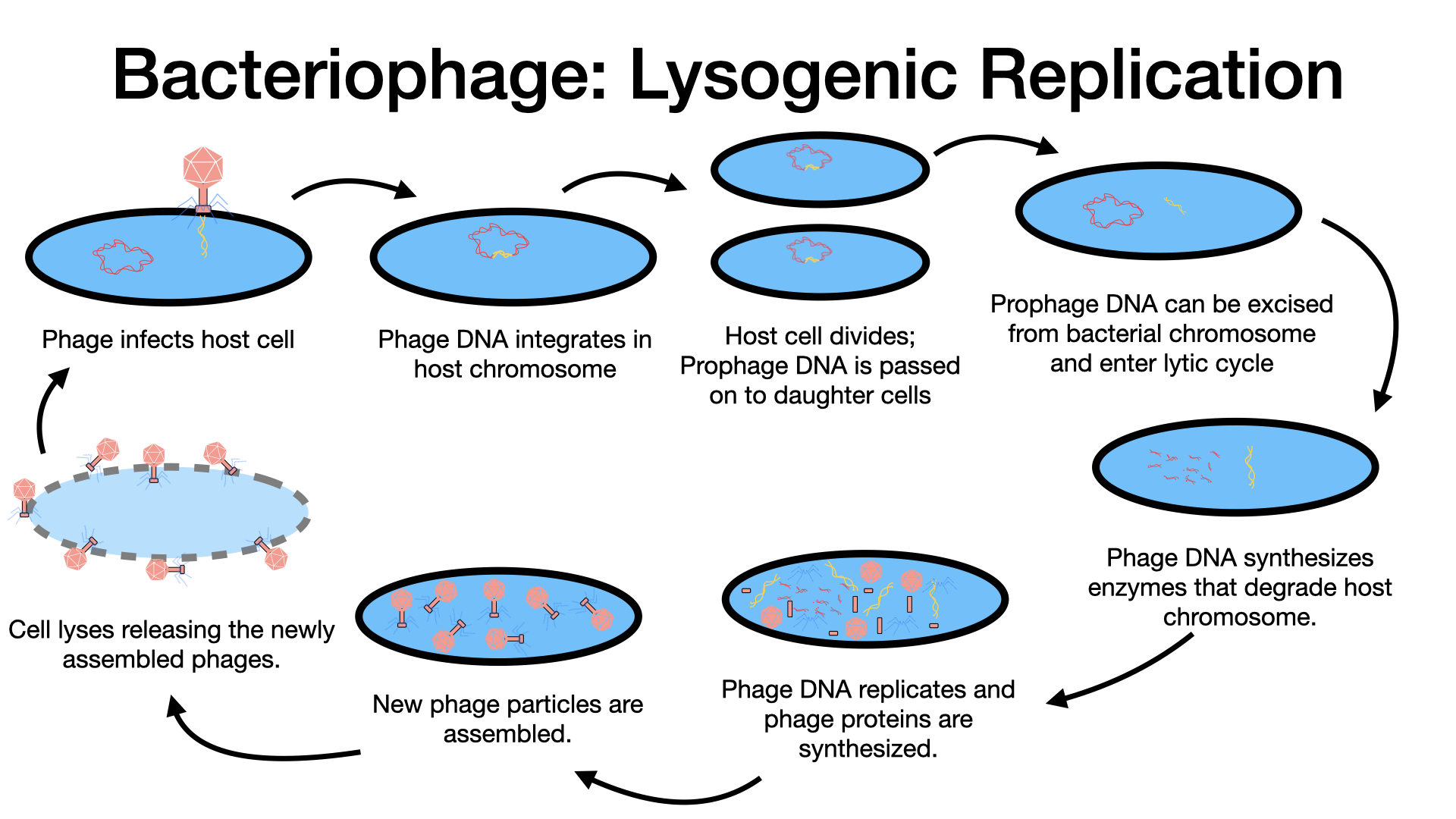
In a lysogenic cycle, the phage genome also enters the cell through attachment and penetration, similar to the lytic cycle. However, instead of immediately hijacking the cell for rapid replication and causing lysis, the phage genome takes a different path. The lambda (λ) phage is a classic example of a temperate phage that can undergo lysogeny.
-
Attachment and Penetration: These initial steps are similar to the lytic cycle, where the phage attaches to the host cell and injects its DNA.
-
Integration (Prophage Formation): This is the hallmark of the lysogenic cycle. Instead of initiating replication of new phage particles, the temperate phage’s DNA integrates into the bacterial chromosome at a specific site. The integrated phage genome is now called a prophage. A bacterial host cell that harbors a prophage is known as a lysogen. The process by which a bacterium is infected by a temperate phage and acquires a prophage is called lysogeny.
-
Replication with Host (Cellular Propagation): Once integrated, the prophage is typically latent or inactive within the cell in terms of producing new virions. As the lysogenic bacterium replicates its own chromosome (e.g., during binary fission), it also replicates the prophage DNA. The prophage is thus passively passed on to all new daughter cells during cell division. In this way, many descendant cells can acquire and carry the viral genome without any new viral particles being produced or any harm coming to the host cells.
-
Lysogenic Conversion (Phage Conversion): The presence of a prophage is not always silent. It can alter the phenotype (observable characteristics) of the host bacterium because the prophage can carry extra genes. These genes might code for new properties, such as toxin production, which can increase the virulence of the bacterium. This change in the host’s phenotype due to the prophage is called lysogenic conversion or phage conversion.
- For example, some bacteria like Vibrio cholerae (which causes cholera) and Clostridium botulinum (which causes botulism) are less virulent in the absence of their respective prophages. The phages infecting these bacteria carry the toxin genes within their genome. When these toxin genes are expressed from the prophage, the virulence of the host bacterium is significantly enhanced. In V. cholerae, the phage-encoded toxin can cause severe diarrhea; in C. botulinum, the toxin can cause paralysis.
-
Induction: The prophage can persist within the host chromosome for many generations. However, under certain conditions, often triggered by environmental stressors (e.g., starvation, exposure to UV radiation or toxic chemicals), the prophage can be induced. Induction results in the excision (cutting out) of the viral genome from the host chromosome. The viral DNA becomes an independent, circular molecule again.
-
Switch to the Lytic Cycle: After induction and excision, the temperate phage can then proceed through a lytic cycle. The now-active viral DNA directs the biosynthesis of new viral components, assembles new virions, and ultimately causes lysis of the host cell, releasing the newly formed phages. These released phages can then infect new host cells, where they might again enter either the lytic or lysogenic cycle, depending on the conditions.
Chapter Summary
Viral replication is entirely dependent on the resources and machinery of a host cell. Viruses infect specific host cells, and their replication strategies vary. Bacteriophages, which infect bacteria, serve as key models for understanding viral life cycles. They can exhibit two primary replication pathways: the lytic cycle and the lysogenic cycle.
- In the lytic cycle, typically employed by virulent phages, the virus rapidly replicates within the host cell, leading to the synthesis of many new viral particles and culminating in the lysis (bursting) and death of the host cell to release the progeny. This cycle involves distinct stages: attachment, penetration, biosynthesis, maturation, and release.
- In the lysogenic cycle, temperate phages can integrate their DNA into the host bacterium’s chromosome, forming a prophage. The host cell, now a lysogen, can replicate normally, passing the prophage to its descendants. This integrated prophage can confer new properties to the bacterium through lysogenic conversion. Under certain conditions (induction), the prophage can excise itself from the host chromosome and enter the lytic cycle, leading to the production and release of new phages.
Understanding these viral replication strategies is crucial for fields ranging from medicine (e.g., understanding pathogenesis and developing antiviral therapies) to biotechnology (e.g., phage therapy).
Media Attributions
- Lytic Cycle Image.001
- Lysogeny.001

