Open Resources for Nursing (Open RN)
Metabolism
After a drug has been absorbed and distributed throughout the body, it is broken down by a process known as metabolism so that it can be excreted from the body. Drugs undergo chemical alteration by various body systems to create compounds that are more easily excreted.
As previously discussed in this chapter, medications that are swallowed or otherwise administered into the gastrointestinal tract are inactivated by the intestines and liver, known as the first-pass effect. Additionally, everything that enters the bloodstream, whether swallowed, injected, inhaled, absorbed through the skin, or produced by the body itself, is metabolized by the liver. See Figure 1.5[1] for an image of the liver. These chemical alterations are known as biotransformations. The biotransformations that take place in the liver are performed by liver enzymes.
Biotransformations occur by mechanisms categorized as either Phase I (modification), Phase II (conjugation), and in some instances, Phase III (additional modification and excretion.)[2]
Phase I biotransformations alter the chemical structure of the drug. Many of the products of enzymatic breakdown, called metabolites, are less chemically active than the original molecule. For this reason, the liver is referred to as a “detoxifying” organ. An example of a Phase I biotransformation is when diazepam, a medication prescribed for anxiety, is transformed into desmethyldiazepam and then to oxazepam. Both these metabolites produce similar physiological and psychological effects of diazepam.[3]
In some instances, Phase I biotransformations change an inactive drug into an active form called a “prodrug.” Prodrugs improve a medication’s effectiveness. They may also be designed to avoid certain side effects or toxicities. For example, sulfasalazine is a medication prescribed for rheumatoid arthritis. It is prodrug that is not active in its ingested form but becomes active after Phase I modification.
Phase II biotransformations involve reactions that couple the drug molecule with another molecule in a process called conjugation. Conjugation typically renders the compound pharmacologically inert and water-soluble so it can be easily excreted. These processes can occur in the liver, kidney, lungs, intestines, and other organ systems. An example of Phase II metabolism is when oxazepam, the active metabolite of diazepam, is conjugated with a molecule called glucuronide so that it becomes physiologically inactive and is excreted without further chemical modification.[4]
Following Phase II metabolism, Phase III biotransformations may also occur, where the conjugates and metabolites are excreted from cells.[5]
Factors Affecting Metabolism
Critical factors in drug metabolism are the type and concentration of liver enzymes. The most important enzymes for medical purposes are monoamine oxidase and cytochrome P450. These two enzymes are responsible for metabolizing dozens of chemicals.[6]
Drug metabolism can be influenced by a number of factors. One major disruptor of drug metabolism is depot binding. Depot binding is the coupling of drug molecules with inactive sites in the body, resulting in the drug not being accessible for metabolism. This action can also affect the duration of action of other medications susceptible to depot binding. For example, tetrahydrocannabinol (THC), the main psychoactive component of marijuana, is highly lipid-soluble and depot binds in the adipose tissue of users. This interaction drastically slows the metabolism of the drug, so metabolites of THC can be detected in urine weeks after the last use.[7]
Another factor in drug metabolism is enzyme induction. Enzymes are induced by repeated use of the same drug. The body becomes accustomed to the constant presence of the drug and compensates by increasing the production of the enzyme necessary for the drug’s metabolism. This contributes to a condition referred to as tolerance and causes clients to require ever-increasing doses of certain drugs to produce the same effect. For example, clients who take opioid analgesics over a long period of time will notice that their medication becomes less effective over time.[8]
In contrast, some drugs have an inhibitory effect on enzymes, making the client more sensitive to other medications metabolized through the action of those enzymes. For example, monoamine oxidase inhibitors (MAOIs) are prescribed as antidepressants because they block monoamine oxidase, the enzyme that breaks down serotonin and dopamine, thus increasing the concentration of these chemicals in the central nervous system. However, this can cause problems when clients taking an MAOI also take other medications that increase the levels of these chemicals, such as dextromethorphan found in cough syrup.[9]
Additionally, drugs that share metabolic pathways can “compete” for the same binding sites on enzymes, thus decreasing the efficiency of their metabolism. For example, alcohol and some sedatives are metabolized by the cytochrome P450 enzyme and only a limited number of these enzymes exist to break these drugs down. Therefore, if a client takes a sedative after drinking alcohol, the sedative is not well-metabolized because most of cytochrome P450 enzymes are filled by alcohol molecules. This results in reduced excretion and high levels of both drugs in the body with enhanced effects. For this reason, the co-administration of alcohol and sedatives can be deadly.
Clinical Significance
When administering medication, nurses must know how and when the medication is metabolized and eliminated from the body. Most of the time, the rate of elimination of a drug depends on the concentration of the drug in the bloodstream. However, the elimination of some drugs occurs at a constant rate that is independent of plasma concentrations. For example, the ethanol contained in alcoholic beverages is eliminated at a constant rate of about 15 mL/hour regardless of the concentration in the bloodstream.[10]
Half Life
Half-life refers to the rate at which 50% of a drug is eliminated from the body. Half-life can vary significantly between drugs. Some drugs have a short half-life of only a few hours and must be given multiple times a day, whereas other drugs have half-lives exceeding 12 hours and can be given as a single dose every 24 hours. See Figure 1.6[11] for an illustration of half-life affecting the blood concentration of medication over time.
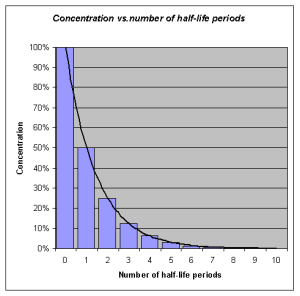
Half-life affects the duration of the therapeutic effect of a medication. Many factors can influence half-life. For example, liver disease can prolong half-life if it is no longer effectively metabolizing the medication. Information about half-life of a specific medication can be found in evidence-based medication references. For example, in the “Clinical Pharmacology” section of the DailyMed reference for furosemide, the half-life is approximately two hours.
Depending on whether a drug is metabolized and eliminated by the kidneys or liver, impairment in either of these systems can significantly alter medication dosing, frequency of doses, anticipated therapeutic effect, and even whether a particular medication can be used at all. Nurses must work with other members of the health care team to prevent drug interactions that could significantly affect a client’s health and well-being. Nurses must be alert for signs of a toxic buildup of metabolites or active drugs, particularly if the client has liver or kidney disease, so that they can alert the health care provider. In other cases, drugs such as warfarin and certain antibiotics are dosed and monitored by pharmacists, who monitor serum levels of the drugs, as well as kidney function.
Life Span Considerations
Neonate & Pediatric
The developing liver in infants and young children produces decreased levels of enzymes. This may result in a decreased ability of the young child or neonate to metabolize medications. In contrast, older children may experience increased metabolism and require higher doses of medications once the hepatic enzymes are fully produced.[12]
Older Adult
Metabolism by the liver may significantly decline in the older adult. As a result, dosages should be adjusted according to the client’s liver function and their anticipated metabolic rate. First-pass metabolism also decreases with aging, so older adults may have higher “free” circulating drug concentrations and thus be at higher risk for side effects and toxicities.[13]
Critical Thinking Activity 1.5
Metabolism can be influenced by many factors within the body. If a client has liver damage, they may not be able to breakdown (metabolize) medications as efficiently. Dosages are calculated according to the liver’s ability to metabolize and the kidney’s ability to excrete.
When caring for a client with cirrhosis, how can this condition impact the dosages prescribed?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” section at the end of the book.
Did You Know?
Did you know that, in some people, a single glass of grapefruit juice can alter levels of drugs used to treat allergies, heart diseases, and infections? Fifteen years ago, pharmacologists discovered this “grapefruit juice effect” by luck, after giving volunteers grapefruit juice to mask the taste of a medicine. Nearly a decade later, researchers figured out that grapefruit juice affects the metabolizing rates of some medicines by lowering levels of a drug-metabolizing enzyme called CYP3A4 (part of the CYP450 family of drug-binding enzymes) in the intestines.
Paul B. Watkins of the University of North Carolina at Chapel Hill discovered that other juices like Seville (sour) orange juice—but not regular orange juice—have the same effect on the liver’s ability to metabolize using enzymes. Each of ten people who volunteered for Watkins’ juice-medicine study took a standard dose of felodopine, a drug used to treat high blood pressure, diluted in grapefruit juice, sour orange juice, or plain orange juice. The researchers measured blood levels of felodopine at various times afterward. The team observed that both grapefruit juice and sour orange juice increased blood levels of felodopine, as if the people had received a higher dose. Regular orange juice had no effect. Watkins and his coworkers have found that a chemical common to grapefruit and sour oranges, dihydroxybergamottin, is likely the molecular culprit. Thus, when taking medications that use the CYP3A4 enzyme to metabolize, clients are advised to avoid grapefruit juice and sour orange juice.[14]

- “Liver Hepatic Organ Jaundice Bile Fatty Liver - Liver” by VSRao is licensed under CC0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- “Concentration_vs_number_of_half-life_periodes.png” by OPPSD is licensed under CC BY-SA 3.0 ↵
- Fernandez, E., Perez, R., Hernandez, A., Tejada, P., Arteta, M., & Ramos, J. T. (2011). Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics, 3(1), 53–72. https://doi.org/10.3390/pharmaceutics3010053 ↵
- Fernandez, E., Perez, R., Hernandez, A., Tejada, P., Arteta, M., & Ramos, J. T. (2011). Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics, 3(1), 53–72. https://doi.org/10.3390/pharmaceutics3010053 ↵
- This work is a derivative of Medicines by Design by US Department of Health and Human Services, National Institutes of Health, National Institute of General Medical Sciences and is available in the Public Domain. ↵
The American Nurses Association (ANA) is a professional organization that represents the interests of the nation's four million registered nurses and is at the forefront of improving the quality of health care for all.[1] The ANA establishes ethical and professional standards for nurses that also guide safe administration of medications. These code of ethics and professional standards are described in ANA publications titled Code of Ethics for Nurses and Nursing: Scope and Standards of Practice.
Code of Ethics for Nurses
The ANA developed the Code of Ethics for Nurses as a guide for carrying out nursing responsibilities in a manner consistent with quality in nursing care and the ethical obligations of the profession.[2] Several provisions from the Code of Ethics impact how nurses should administer medication in an ethical manner. A summary of each provision from the Code of Ethics and how it pertains to medication administration is outlined below:
- Provision 1 focuses on respect for human dignity and the right for self-determination: “The nurse practices with compassion and respect for the inherent dignity, worth, and unique attributes of every person.”
- Provision 2 states, “The nurse's primary commitment is to the client…”[3] In health care settings, nurses often experience several competing loyalties, such as to their employer, to the doctor(s), to their supervisor, or to others on the health care team. However, the client should always receive the primary commitment of the nurse. Additionally, the client has the right to accept, refuse, or terminate any treatment, including medications.
- Provision 3 states, “The nurse promotes, advocates for, and protects the rights, health, and safety of the patient...”[4] This provision includes a nurse's responsibility to promote a culture of safety for clients. If errors occur, they must be reported, and nurses should ensure responsible disclosure of errors to clients. This also includes proper disclosure of questionable practices, such as drug diversion or impaired practice by any professional.
- Provision 4 involves authority, accountability, and responsibility by a nurse to follow legal requirements, such as state practice acts and professional standards of care.
- Provision 5 includes the responsibility of the nurse to promote health and safety.
- Provision 6 focuses on virtues that make a nurse a morally good person. For example, nurses are held accountable to use their clinical judgment to avoid causing harm to clients (maleficence) and to do good (beneficence). When administering medications, nurses should validate the medication is doing more “good” than “harm” (adverse or side effects).
- Provision 7 focuses on a nurse practicing within the professional standards set forth by their state nurse practice act, as well as standards established by professional nursing organizations.
- Provision 8 explains that a nurse must address the social determinants of health, such as poverty, education, safe medication, and health care disparities.[5]
Whenever a nurse provides client care, the ANA's Code of Ethics should be used as a guide for professional ethical behavior.
View the ANA's Code of Ethics for Nurses.
Critical Thinking Activity 2.2a
A nurse is preparing to administer medications to a client. While reviewing the chart, the nurse notices two medications with similar mechanisms of action have been prescribed by two different providers.
What is the nurse's best response?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” sections at the end of the book.
Standards and Scope of Practice
The ANA publishes Nursing: Scope and Standards of Practice. This resource establishes national standards for nurses and is updated regularly.[6]
The ANA defines the scope of nursing as “the protection, promotion, and optimization of health and abilities, prevention of illness and injury, facilitation of healing, alleviation of suffering through the diagnosis and treatment of human response, and advocacy in the care of individuals, families, groups, communities, and populations.” A registered nurse (RN) is defined as an individual who is educationally prepared and licensed by a state to practice as a registered nurse. Nursing practice is characterized by the following tenets[7]:
- Caring and health are central to the practice of the registered nurse.
- Nursing practice is individualized to the unique needs of the health care consumer.
- Registered nurses use the nursing process to plan and provide individualized care for health care consumers.
- Nurses coordinate care by establishing partnerships to reach a shared goal of delivering safe, quality health care.
The ANA establishes Standards of Practice and Standards of Professional Performance in the Nursing: Scope and Standards of Practice publication. State nurse practice acts further define the scope of practice of RNs and Licensed Practical Nurses/Vocational Nurses (LPNs/VNs) within each state. Nurse practice acts are further discussed in the “Legal Foundations and National Guidelines for Safe Medication Administration” section of this chapter.
The ANA's Nursing: Scope and Standards of Practice publication can be purchased on the nursingworld.org website or borrowed from many libraries.
Standards of Practice
The ANA's Standards of Practice are authoritative statements of duties that all registered nurses, regardless of role, population, or specialty, are expected to perform competently. Standards of Practice include assessment, diagnosis, outcome identification, planning, implementation, and evaluation (ADOPIE) components of providing client care, also known as the "nursing process." When nurses safely administer medication, all components of ADOPIE are addressed.
Assessment
The "Assessment" Standard of Practice is defined as, “The registered nurse collects pertinent data and information relative to the health care consumer’s health or the situation.”[8] A registered nurse uses a systematic method to collect and analyze client data. Assessment includes physiological data, as well as psychological, sociocultural, spiritual, economic, and lifestyle data. For example, when a nurse assesses multiple pieces of data for a hospitalized client with pain, this is considered part of a comprehensive pain assessment.
Diagnosis
The "Diagnosis" Standard of Practice is defined as, “The registered nurse analyzes the assessment data to determine actual or potential diagnoses, problems, and issues.”[9] A nursing diagnosis is the nurse’s clinical judgment about the client's response to actual or potential health conditions or needs. Nursing diagnoses are used to create the nursing care plan and are different than medical diagnoses.[10]
Outcomes Identification
The "Outcomes Identification" Standard of Practice is defined as, “The registered nurse identifies expected outcomes for a plan individualized to the health care consumer or the situation.”[11] The nurse sets measurable and achievable short- and long-term goals and specific outcomes in collaboration with the client based on their assessment data and nursing diagnoses.
Planning
The "Planning" Standard of Practice is defined as, “The registered nurse develops a collaborative plan encompassing strategies to achieve expected outcomes.”[12] Assessment data, diagnoses, and goals are used to select evidence-based nursing interventions customized to each client’s needs and concerns. Goals, expected outcomes, and nursing interventions are documented in the client’s nursing care plan so that nurses, as well as other health professionals, have access to it for continuity of care.[13]
Implementation
The "Implementation" Standard of Practice is defined as, "The nurse implements the identified plan.”[14] Nursing interventions are implemented or delegated to licensed practical nurses/vocational nurses (LPNs/VNs) or unlicensed assistive personnel (UAP) with supervision. Interventions are also documented in the client’s electronic medical record as they are completed.[15]
The "Implementation" Standard of Professional Practice also includes the subcategories "Coordination of Care" and "Health Teaching and Health Promotion" to promote health and a safe environment.[16]
Coordination of Care
The ANA standard for coordination of care states, “The registered nurse coordinates care delivery.”[17] When ensuring medications are administered safely, the nurse collaborates with the client and the interprofessional health care team to meet mutually agreed upon outcomes. The nurse also engages the client in self-care to achieve their preferred goals for quality of life. For example, one client with chronic pain may have a pain management goal of "5" with their quality of life preference of having the ability to participate in social activities with friends but not experiencing burdensome side effect of medication. Another client with chronic pain may have a pain management goal of "0" with a quality of life preference of having no pain no matter what the side effects. The nurse advocates for these clients' goals and preferences with the interprofessional team.
Nurses also serve vital roles in ensuring safe transitions and continuity of care regarding clients' use of medications. Additional information about safe medication use and transitions of care is discussed in the "Preventing Medication Errors" section of this chapter.
Health Teaching and Health Promotion
When administering medications, nurses teach clients about the medications and potential side effects to promote optimal health. The ANA standard for health teaching and health promotion states, “The registered nurse employs strategies to teach and promote health and wellness.”[18] Specific behaviors related to teaching about medication are as follows[19]:
- Use health teaching and health promotion methods in collaboration with the client's values, beliefs, health practices, developmental level, learning needs, readiness and ability to learn, language preference, spirituality, culture, and socioeconomic status.
- Provide clients with information and education about intended effects and potential adverse effects of the plan of care.
- Provide anticipatory guidance to clients to promote health and prevent or reduce risk.
In the book Preventing Medication Errors by the Institute of Medicine (2007), the following are additional key national guidelines when teaching clients about safe use of their medications:
- Clients should maintain an active list of all prescription drugs, over-the-counter (OTC) drugs, and dietary supplements they are taking, the reasons for taking them, and any known drug allergies. Every provider involved in the medication-use process for a client should have access to this list.
- Clients should be provided information about side effects, contraindications, methods for handling adverse reactions, and sources for obtaining additional objective, high-quality information.[20]
Evaluation
The "Evaluation" Standard of Practice is defined as, “The registered nurse evaluates progress toward attainment of goals and outcomes.”[21] During evaluation, nurses assess the client and compare the findings against the initial assessment to determine the effectiveness of the interventions and overall nursing care plan. Both the client’s status and the effectiveness of the nursing care must be continuously evaluated and modified as needed.[22]
Read additional information about the nursing process in the "Nursing Process" chapter of Open RN Nursing Fundamentals.
Standards of Professional Performance
ANA's Standards of Professional Performance describe a competent level of behavior for nurses, including activities related to ethics, culturally congruent practice, communication, collaboration, leadership, education, evidence-based practice, and quality of practice.[23]
The ANA defines culturally congruent practice as the application of evidence-based nursing that is in agreement with the preferred cultural values, beliefs, worldview, and practices of the health care consumer and other stakeholders. Cultural competence represents the process by which nurses demonstrate culturally congruent practice. Nurses must assess the cultural beliefs and practices of their clients and implement culturally congruent interventions when administering medications and teaching about them. Additional information about cultural implications for medication administration is further discussed in the “Cultural and Social Determinants Related to Medication Administration” section later in this chapter.
Critical Thinking Activity 2.2b

A nurse is preparing to administer metoprolol, a cardiac medication, to a client and implements the nursing process:
ASSESSES the vital signs prior to administration and discovers the heart rate is 48.
DIAGNOSES that the heart rate is too low to safely administer the medication per the parameters provided. Establishes the OUTCOME to keep the client's heart rate within normal range of 60-100.
PLANS to call the provider, as well as report this incident in the shift handoff report.
Implements INTERVENTIONS by withholding the metoprolol at this time, documenting the incident that the medication is withheld, and notifying the provider.
Continues to EVALUATE the client status throughout the shift after not receiving the metoprolol.
The nurse is providing health teaching to a client about the medication before discharge. The nurse provides a handout with instructions, as well as a list of the current medications.
What other information should be provided to the client?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” sections at the end of the book.
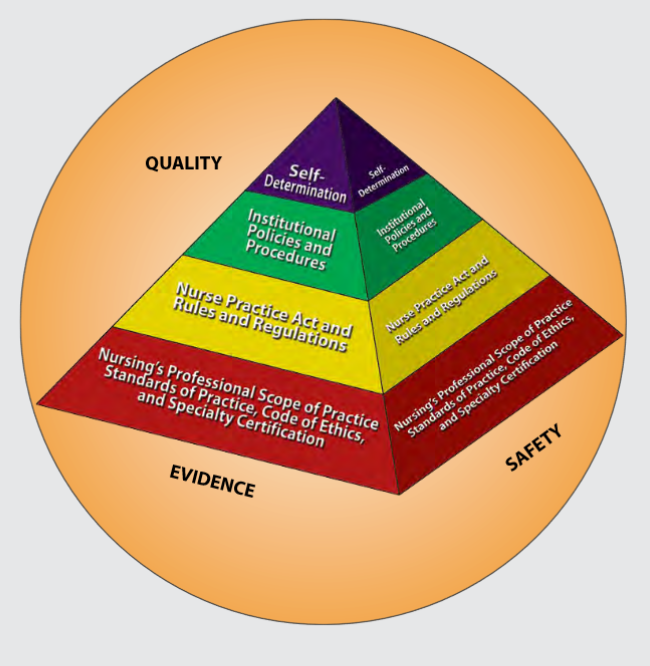
Figure 2.1 is an image from Nursing: Scope and Standards of Practice by the ANA that illustrates how the scope of practice, standards of practice, and code of ethics form the “base” of nursing practice.[24] Nursing practice is further guided by the Nurse Practice Act in the state in which a nurse works, federal and state rules and regulations, institutional policies and procedures, and self-determination by the individual nurse. All these components are required to provide quality, safe client care that is evidence-based. These components will be further discussed in the remaining sections of this chapter.
NCLEX and the Clinical Judgment Model
The National Council Licensure Examination (NCLEX) is the national exam that graduates must pass successfully to obtain their nursing license after graduating from a nursing program of study. The NCLEX-PN is taken to become a licensed practical/vocational nurse (LPN/VN), and the NCLEX-RN is taken to become a licensed registered nurse (RN). The purpose of the NCLEX is to evaluate if a nursing graduate demonstrates the ability to provide safe, competent, entry-level nursing care. The NCLEX is developed by the National Council of State Boards of Nursing (NCSBN), an independent, nonprofit organization composed of the 50 state boards of nursing and other regulatory agencies.[25]
A new edition of the NCLEX was launched in April 2023 that contains “Next Generation” questions. The Next Generation NCLEX (NGN) assesses how well the candidate can think critically and use clinical judgment. The NCSBN defines clinical judgment as "the observed outcome of critical thinking and decision-making. It is an iterative process with multiple steps that uses nursing knowledge to observe and assess presenting situations, identify a prioritized client concern and generate the best possible evidence-based solutions in order to deliver safe client care."
The NCLEX uses the NCSBN's Clinical Judgment Measurement Model (NCJMM) to assess the candidate's ability to use safe clinical judgment when providing nursing care. Exam questions used to assess clinical judgment may be contained in a case study or as individual stand-alone items. A case study contains six questions that are associated with the same client scenario and addresses the following steps in clinical judgment[26]:
- Recognize cues: Identify relevant and important information from different sources (e.g., medical history, vital signs).
- Analyze cues: Organize and connect the recognized cues to the client’s clinical presentation.
- Prioritize hypotheses: Evaluate and prioritize hypotheses (based on urgency, likelihood, risk, difficulty, time constraints, etc.).
- Generate solutions: Identify expected outcomes and use hypotheses to define a set of interventions for the expected outcomes.
- Take action: Implement the solution(s) that address the highest priority.
- Evaluate outcomes: Compare observed outcomes to expected outcomes.
Throughout this book, learning activities are provided to assist students in learning how to apply the nursing process (i.e., ANA's Standards of Care) to answer NGN-style questions that evaluate clinical judgment. Some of these activities are written, with answers in the Answer Key at the end of the book, and others are interactive and require use of the online book.

