Chapter 3b: Part 2 Ecosystems – Energy and Biogeochemical Cycles
3.6 Case Study: Eutrophication in Chesapeake Bay
Learning Outcomes:
After studying chapter 3b, each student should be able to:
- 3.2 Follow energy through an ecosystem by both analyzing and creating food chains and food webs for various ecosystems
- 3.3 Describe how organisms first acquire energy in a ecosystem and follow that energy through various food webs
- 3.4 Discuss the concept of trophic levels in terms of producers, secondary and tertiary consumers, detrivores and other related food producers and users
- 3.5 Discuss the biogeochemical cycles of water, carbon, nitrogen, phosphorus, and sulfur, and explain how human activities have impacted these cycles
- 3.6 Understand how the Chesapeake Bay ecosystem was degraded due to eutrophication and what is being done to remedy this situation
3.2 Energy and Ecosystems
As you learned about the ecosystems and biomes, you may have noticed an emphasis placed on the amount of sunlight available for photosynthesis. This is because photosynthesis is how energy enters most ecosystems. Before you learn more about photosynthesis and how energy is passed from one organism to another, you first need to learn what energy is.
Energy
In general, energy is defined as the ability to do work, or to create some kind of change. Virtually every task performed by living organisms requires energy. For example, energy is required for the synthesis and breakdown of molecules, as well as the transport of molecules into and out of cells. In addition, processes such as ingesting and breaking down food, exporting wastes and toxins, and movement of the cell all require energy. Nutrients and other molecules are imported into cells to meet these energy demands.
Scientists use the term bioenergetics to describe the concept of energy flow through living systems, such as cells. Cellular processes such as the building and breaking down of complex molecules occur through multiple chemical reactions. Some of these chemical reactions release energy, whereas others require energy to proceed. Together, all of the chemical reactions that take place inside cells are referred to as the cell’s metabolism.
Thermodynamics refers to the study of energy and energy transfer involving physical matter. The matter relevant to a particular case of energy transfer is called a system, and everything outside of that matter is called the surroundings. For instance, when heating a pot of water on the stove, the system includes the stove, the pot, and the water. Energy is transferred within the system (between the stove, pot, and water). There are two types of systems: open and closed. In an open system, energy can be exchanged with its surroundings. The stovetop system is open because heat can be lost to the air. A closed system cannot exchange energy with its surroundings.
Biological organisms are open systems. Energy is exchanged between them and their surroundings as they use energy from the sun to perform photosynthesis or consume energy-storing molecules and release energy to the environment by doing work and releasing heat. Like all things in the physical world, energy is subject to physical laws. The laws of thermodynamics govern the transfer of energy in and among all systems in the universe. To appreciate the way energy flows into and out of biological systems, it is important to understand two of the physical laws that govern energy.
The First and Second Laws of Thermodynamics
The first law of thermodynamics states that the total amount of energy in the universe is constant and conserved. In other words, there has always been, and always will be, exactly the same amount of energy in the universe. Energy exists in many different forms, such as electrical energy, light energy, mechanical energy, and heat energy. According to the first law of thermodynamics, energy may be transferred from place to place or transformed into different forms, but it cannot be created or destroyed. The transfers and transformations of energy take place around us all the time. Light bulbs transform electrical energy into light and heat energy. Gas stoves transform chemical energy from natural gas into heat energy. Plants perform one of the most biologically useful energy transformations on earth: that of converting the energy of sunlight to chemical energy stored within organic molecules through the process of photosynthesis (Figure 1).
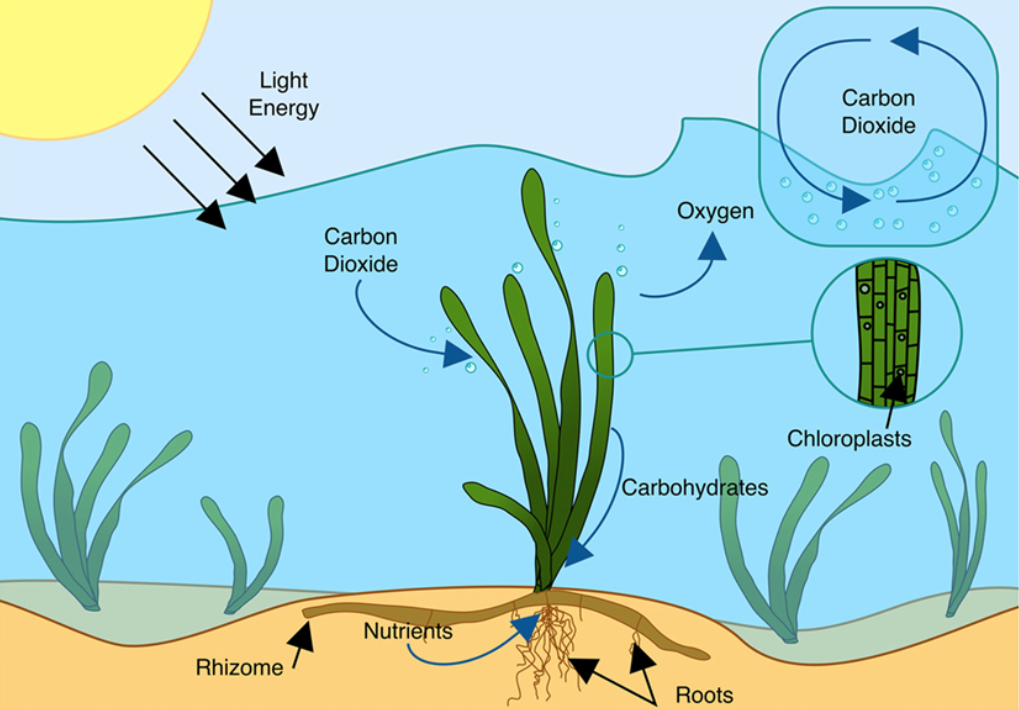
Figure 1. Photosynthetic organisms such as seagrass can convert the light energy of the sun into carbohydrates, a molecular form of chemical energy. (Credit: Attribution: Cullen-Unsworth L., Jones, B., Lilley, R. and Unsworth, R.; Licensed under the Creative Commons Attribution 4.0 International license)
The challenge for all living organisms is to obtain energy from their surroundings in forms that are usable to perform cellular work. Chemical energy stored within organic molecules such as sugars and fats is transferred and transformed through a series of cellular chemical reactions into energy stored in molecules of ATP (adenosine triphosphate). Energy in ATP molecules is easily accessible by cells to do work. Examples of the types of work that cells need to do include building complex molecules, transporting materials, powering the motion of cilia or flagella, and contracting muscles to create movement. You can think of ATP as a rechargeable battery that is depleted as a cell does work. It can be recharged when cells extract the energy stored in organic compounds through the process of respiration.
A living cell’s primary tasks of obtaining, transforming, and using energy to do work may seem simple. However, the second law of thermodynamics explains why these tasks are harder than they appear. The second law of thermodynamics states that all energy transfers and transformations are never completely efficient, with some energy lost in an unusable form. In most cases, the energy lost by living things during energy transfer is lost in the form is heat energy. For example, when a light bulb is turned on, some of the energy being converted from electrical energy into light energy is lost as heat energy. Likewise, some energy is lost as heat energy during cellular metabolic reactions.
Potential and Kinetic Energy
As mentioned previously, energy can exist in different forms. The two main forms of energy are potential energy and kinetic energy. When an object is in motion, there is energy associated with that object. Think of a wrecking ball. Even a slow-moving wrecking ball can do a great deal of damage to other objects. Energy associated with objects in motion is called kinetic energy. A speeding bullet, a walking person, and the rapid movement of molecules in the air all have kinetic energy.
Now what if that same motionless wrecking ball is lifted two stories above ground with a crane? If the suspended wrecking ball is not moving, is there energy associated with it? The answer is yes. The energy that was required to lift the wrecking ball did not disappear but is now stored in the wrecking ball by virtue of its position and the force of gravity acting on it. This type of energy is called potential energy. If the ball were to fall, the potential energy would be transformed into kinetic energy until all of the potential energy was exhausted when the ball rested on the ground. Other examples of potential energy include the energy of water held behind a dam or a person about to skydive out of an airplane.
Potential energy is not only associated with the location of matter, but also with the structure of matter. Even a spring on the ground has potential energy if it is compressed; so does a rubber band that is pulled taut. On a molecular level, the bonds that hold the atoms of molecules together exist in a particular structure that has potential energy. The fact that energy can be released by the breakdown of certain chemical bonds implies that those bonds have potential energy. In fact, there is potential energy stored within the bonds of all the food molecules we eat, which is harnessed for use. The type of potential energy that exists within chemical bonds of molecules, and is released when those bonds are broken, is called chemical energy. Chemical energy is responsible for providing living cells with energy from food. The release of energy occurs when the molecular bonds within food molecules are broken.
3.3 How Organisms Acquire Energy
All living things require energy in one form or another, however, organisms can acquire energy in a variety of ways. Photosynthetic and chemosynthetic organisms are autotrophs, which are organisms capable of synthesizing their own food (more specifically, capable of using inorganic carbon as a carbon source). Photosynthetic autotrophs (photoautotrophs) use sunlight as an energy source, and chemosynthetic autotrophs (chemoautotrophs) use inorganic molecules as an energy source. Autotrophs are critical for ecosystems because they occupy the trophic level containing producers. Without these organisms, energy would not be available to other living organisms, and life would not be possible.
Chemoautotrophs are primarily bacteria and archaea that are found in rare ecosystems where sunlight is not available, such as those associated with dark caves or hydrothermal vents at the bottom of the ocean (Figure 2). Many chemoautotrophs in hydrothermal vents use hydrogen sulfide (H2S), which is released from the vents, as a source of chemical energy. This allows them to synthesize complex organic molecules, such as glucose, for their own energy and, in turn, supplies energy to the rest of the ecosystem.

Figure 2. Hydrothermal vents and associated tube worms being studied by scientists. These worms are in the phylum Annelida, like the common earthworm, but get their energy from chemosynthetic bacteria living within them. These bacteria use the sulfur and heat of the vents to produce food. (Credit: This image is in the public domain; image contains materials that originally came from the U.S. National Oceanic and Atmospheric Administration, taken or made as part of an employee’s official duties.)
Photoautotrophs, such as plants, algae, and photosynthetic bacteria, are the energy source for the majority of the world’s ecosystems. Through photosynthesis, these organisms convert solar energy (sunlight) into chemical energy, which is then used to build carbohydrate molecules. The rate at which photosynthetic producers incorporate energy from the sun is called gross primary productivity. However, not all of the energy incorporated by producers is available to the other organisms in the food web because producers must use some of the energy themselves to grow and reproduce. Net primary productivity is the energy that remains in the producers after accounting for these organisms’ metabolism and heat loss. The net productivity is then available for other organisms to use.
Heterotrophs are organisms incapable of photosynthesis that must therefore obtain energy and carbon from food by consuming other organisms. The Greek roots of the word heterotroph mean “other” (hetero) “feeder” (troph), meaning that their food comes from other organisms. Humans are heterotrophs, as are all animals and fungi. Heterotrophs depend on autotrophs, either directly or indirectly, or energy. For example, a deer obtains energy by eating plants. A wolf eating a deer obtains energy that originally came from the plants eaten by that deer (Figure 2). Using this reasoning, all of the food eaten by heterotrophs can be traced back to autotrophs, with the majority coming from photoautotrophs.
Summary of Photosynthesis
Photosynthesis requires sunlight (energy), carbon dioxide (CO2), and water (H2O) as starting reactants (Figure 3). After the process is complete, photosynthesis releases oxygen gas (O2) and produces carbohydrate (sugar) molecules. These sugar molecules contain the energy that living things need to survive. The complex reactions of photosynthesis can be summarized by the chemical equation shown in figure 17.

Figure 3. This equation means that six molecules of carbon dioxide (CO2) combine with six molecules of water (H2O) in the presence of sunlight. This produces one molecule of glucose (C6H12H12) and six molecules of oxygen (O2). (Credit: The author, ZooFari, has placed this image in the Public Domain to use for any purpose, without conditions.)
Although the photosynthetic equation looks simple, the many steps that take place during photosynthesis are actually quite complex and require specialized structures to occur. In plants, photosynthesis takes place primarily in the chloroplasts of leaves. Chloroplasts are complex organelles that contain the pigment, chlorophyll, which absorbs energy from the Sun to start the process of photosynthesis.
The following video will help you understand the process of photosynthesis better:
Neurotech Lectures. (2019, May 10). How Photosynthesis Takes Place in Plants [Video – YouTube] https://youtu.be/xEF8shaU_34
The Global Significance of Photosynthesis
In addition to capturing energy for ecosystems, the process of photosynthesis is also crucially important to the biosphere because it creates oxygen. Photosynthetic bacteria were likely the first organisms to perform photosynthesis, dating back 2-3 billion years. Thanks to their activity, and a diversity of present-day photosynthesizing organisms, Earth’s atmosphere is currently about 21% oxygen gas (O2).
The creation of atmospheric oxygen (O2) by photosynthesis is important for two reasons.
It is needed to support cellular respiration, which are the chemical reactions that allow organisms to convert the energy stored in chemical bonds to ATP.
It is vital for the creation of the ozone layer, which protects life from harmful ultraviolet radiation emitted by the sun. Ozone (O3) is created from the breakdown and reassembly of O2.
Additionally, photosynthesis provides the carbon needed to produce organic molecules. Organisms are primarily made of two things: water and organic molecules. Organic molecules are those that contain the elements carbon and hydrogen. Through the process of carbon fixation, photosynthesis takes carbon from carbon dioxide gas (CO2) in the atmosphere and converts it into sugars (which are organic compounds). Carbon in these sugars can be repurposed to create other types of organic molecules that organisms need, such as lipids, proteins, and nucleic acids. For example, the carbon used to make your DNA was once CO2 used by photosynthetic organisms for photosynthesis. Therefore, photosynthesis is vitally important for the production of biomass.
3.4 Trophic Levels
As previously mentioned, heterotrophs must eat other organisms to obtain energy. The flow of energy from one organism to another can be illustrated in the form of a food chain or a food web. A food chain is a linear sequence of organisms through which nutrients and energy pass as one organism eats another. The levels in the food chain are producers, primary consumers, higher-level consumers (secondary consumers, tertiary consumers, etc.), and finally, decomposers. These levels are used to describe ecosystem structure and dynamics. Food chains show a simplistic, linear model of ecosystem dynamics with each organism in a food chain only eating one other type of organism and occupying a specific trophic level (energy level).
In many ecosystems, the base, or foundation, of the food chain consists of photosynthetic organisms (plants, algae or phytoplankton), which are called producers. The organisms that consume the producers are primary consumers. Primary consumers are also called herbivores, because they only eat plant material. Secondary consumers are carnivores (animals that eat the primary consumers). Tertiary consumers are carnivores that eat other carnivores and are fairly rare, except in aquatic biomes, where larger fish eat smaller fish. Higher-level consumers feed on the next lower trophic levels, and so on, up to the organisms at the top of the food chain. In the food pyramid shown below (Figure 4), one can note that the producers provide the food that all other members of this food chain ultimately depend on.
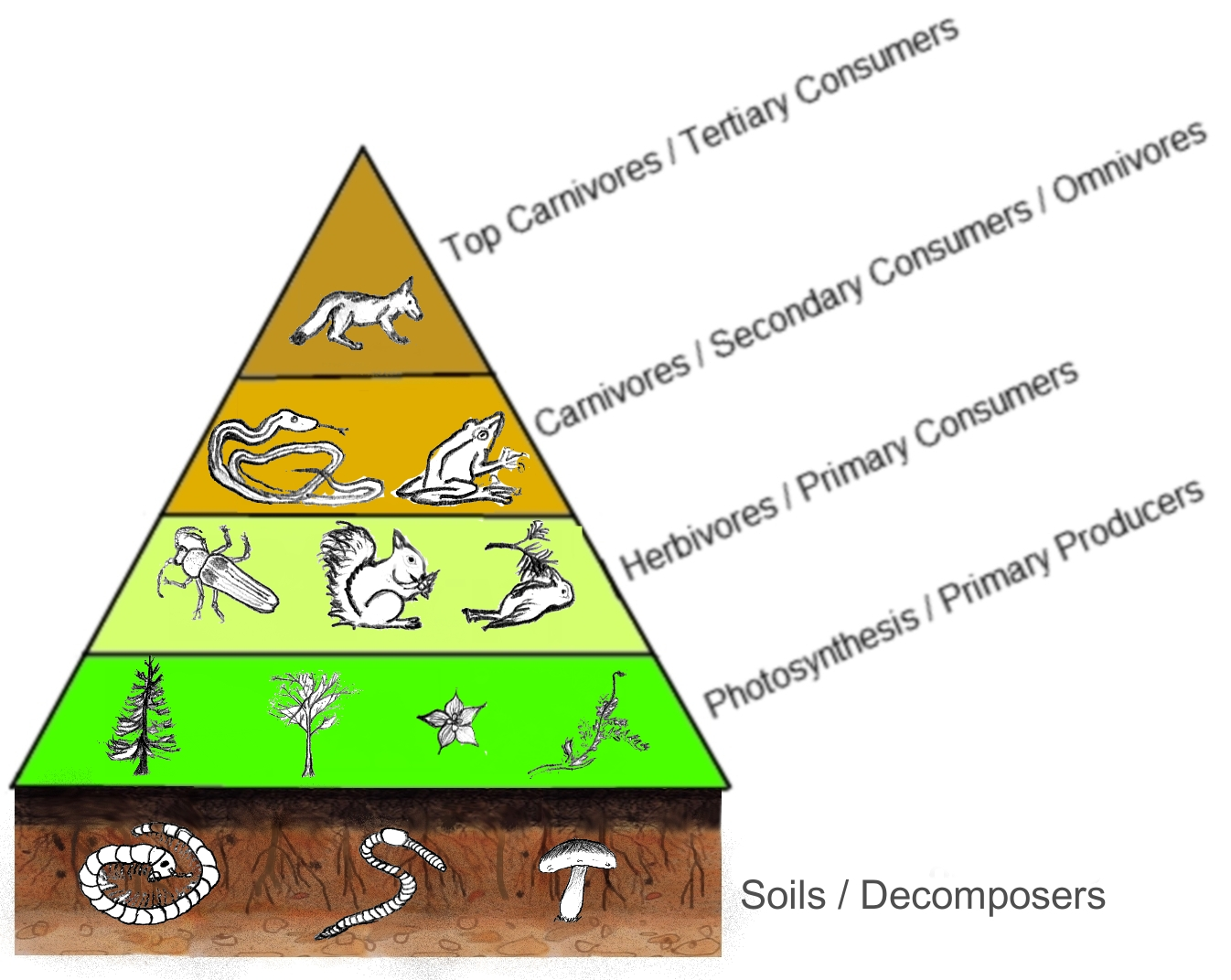
Figure 4. A food pyramid that might be found in a forest ecosystem, showing a number of producers, various levels of consumers, and some common decomposers. (Credit: Author, Thompsma; This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license)
One major factor that limits the number of steps in a food chain is energy loss that occurs due to the second law of thermodynamics. Heat energy is lost to the environment as organisms at a particular trophic level perform respiration and during energy transfers between trophic levels. Generally speaking, only about 10% of the energy at one trophic level is successfully transferred to the next trophic level, with 90% of the energy lost as heat. For example, if there are 1000 units of energy in a producer, only 100 units of that energy will actually be available to the primary consumer (herbivore) that ate the producer. When the herbivore is eaten by a secondary consumer (carnivore), only 10 units of the original 1000 units of energy will be available to the secondary consumer. Thus, after a limited number of trophic energy transfers, the amount of energy remaining in the food chain may not be great enough to support viable populations at higher trophic levels.
Food chains are relatively simplistic models when describing the flow of biomass and energy through an ecosystem. Some organisms can feed at more than one trophic level. For example, omnivores, organisms that eat both meat and plant matter, may feed at the primary and secondary consumer levels. In addition, species feed on and are eaten by more than one species. In other words, the linear model of a food chain is an overly simplistic representation of ecosystem structure. A food web includes all the interactions between different species (all food chains!) and their complex interconnected relationships with each other and with the environment and is a more accurate and descriptive model for ecosystems. Food webs account for the multiple trophic (feeding) interactions between each species (Figure 5).
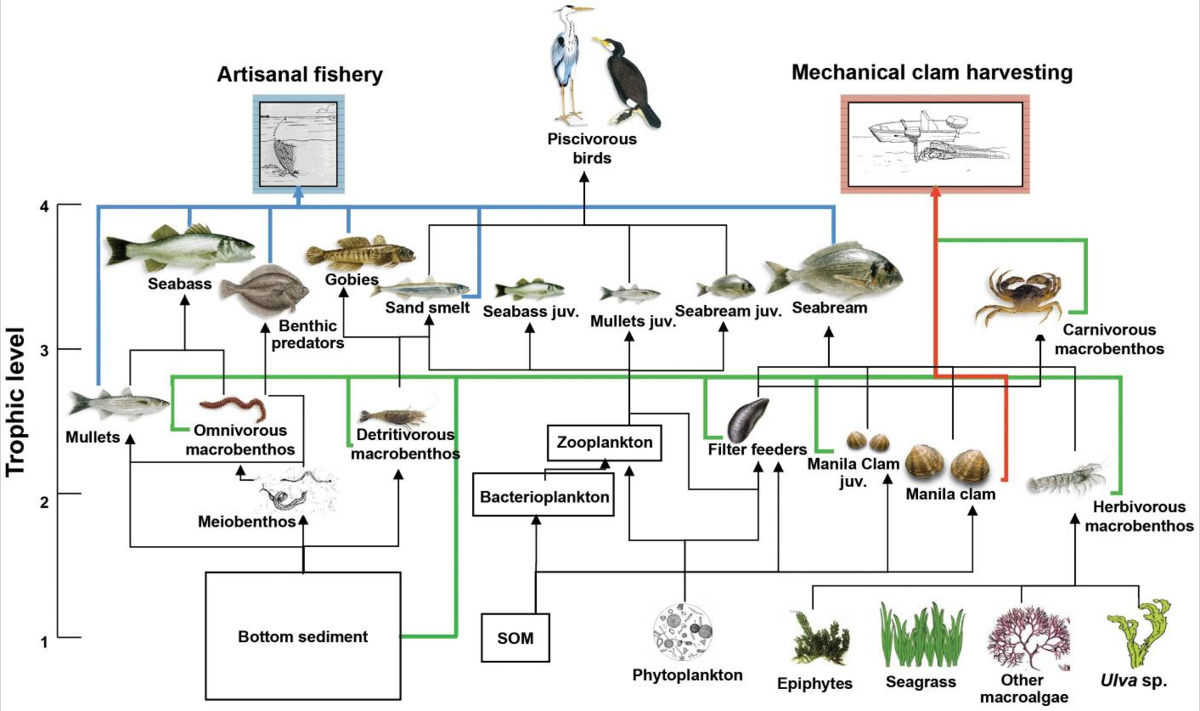
Figure 5. The food web found in a Venice lagoon. A food web shows the many organisms that may be at each trophic level, and the variety of ways that energy may pass between feeding levels. (Credit:Authors, Heymans, J.J., Coll, M., Libralato, S., Morissette, L. and Christensen, V.; This file is licensed under the Creative Commons Attribution 4.0 International license.)
Food chains and food webs often show energy and biomass being transferred in one direction, but in reality, trophic interactions are a cycle. When organisms die, whether they are producers or consumers, their biomass and energy are recycled back into the system by decomposers, which break down dead and decaying organisms, and detritivores, which consume organic detritus. These organisms are usually bacteria, fungi, and invertebrate animals that recycle organic material back into the biotic part of the ecosystem as they themselves are consumed by other organisms. The material in the soil can then be used to support the growth of new producers.
Biomagnification
One of the most important consequences of ecosystem dynamics in terms of human impact is biomagnification. Biomagnification is the increasing concentration of persistent, toxic substances in organisms at each successive trophic level. Substances biomagnify if they are lipid-soluble, meaning they are stored in an organism’s fat and not excreted like water-soluble compounds. As an organism eats more and more contaminated food, more and more of the compound gets deposited in the organism’s fat. Organisms feeding at higher trophic levels are accumulating these toxic compounds from their food, which accumulated toxic compounds from their food. These compounds, therefore, are more likely to negatively affect organisms feeding higher up the food chain.
Many substances have been shown to biomagnify, including classical studies with the pesticide dichlorodiphenyltrichloro-ethane (DDT), which were described in the 1960s bestseller Silent Spring by Rachel Carson. DDT was a commonly used pesticide before its dangers to apex consumers, such as the bald eagle, became known. As bald eagles feed on contaminated fish, their DDT levels rise. It was discovered that DDT caused the eggshells of birds to become fragile, which contributed to the bald eagle being listed as an endangered species under U.S. law. The use of DDT was banned in the United States in the 1970s.
Another substance that biomagnifies is polychlorinated biphenyl (PCB), which was used as coolant liquids in the United States until its use was banned in 1979. PCB was best studied in aquatic ecosystems where predatory fish species accumulated very high concentrations of the toxin that otherwise exists at low concentrations in the environment. PCB concentrations increased from the producers of the ecosystem (phytoplankton) through the different trophic levels of fish species. The apex consumer, the walleye, has more than four times the amount of PCB compared to phytoplankton. Also, research found that birds that eat these fish may have PCB levels that are at least ten times higher than those found in the lake fish.
Other concerns have been raised by the biomagnification of heavy metals, such as mercury and cadmium, in certain types of seafood. The United States Environmental Protection Agency (EPA) recommends that pregnant women and young children should not consume any swordfish, shark, king mackerel, or tilefish because of their high mercury content. These individuals are advised to eat fish low in mercury: salmon, shrimp, pollock, and catfish. Biomagnification is a good example of how ecosystem dynamics can affect our everyday lives, even influencing the food we eat.
3.5 Biogeochemical Cycles
Energy is not the only substance transferred around ecosystems. Matter, in the form of atoms and molecules, is also transferred from one organism to another and between the biotic and abiotic realms. As an individual organism, you are essentially “borrowing” the matter that makes up your body. The atoms that make up your cells once belonged to the organisms that you ate. When you die, your body will decompose and your atoms will return to the Earth for other organisms to use.
The most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, and phosphorus—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth’s surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of these processes, they are called biogeochemical cycles. These cycles describe the recycling of inorganic matter between living organisms and their environment. Although interconnected, the various biogeochemical cycles can be studied somewhat independently. For example water, nitrogen, and phosphorous can be studied as they move and are converted within the environment. As with many components of the environment, humans have used technology to alter these cycles, leading to some negative environmental impacts.
Water contains hydrogen and oxygen, which are essential to life. Carbon is found in all organic macromolecules and is an important constituent of fossil fuels. Nitrogen is a major component of our nucleic acids and proteins and is critical to human agriculture. Phosphorus, a major component of nucleic acids (along with nitrogen), is one of the main ingredients in artificial fertilizers used in agriculture and their associated environmental impacts on our surface water. The cycling of these elements is interconnected. For example, the movement of water is critical for the leaching of nitrogen and phosphate into rivers, lakes, and oceans. Furthermore, the ocean itself is a major reservoir for carbon. Thus, mineral nutrients are cycled, either rapidly or slowly, through the entire biosphere, from one living organism to another, and between the biotic and abiotic world..
Although interconnected, the cycles can be studied somewhat independently to follow the movement and conversion of water, nitrogen, and phosphorus throughout the environment. As with many components of the environment, humans have used technology to alter these cycles, leading to negative environmental impacts.
The following video by Bozeman Science gives an easy to understand overview of the biogeochemical cycles in nature:
Bozeman Science. (2015, Sept 28). Biogeochemical Cycles [Video – YouTube] https://youtu.be/Bn41lXKyVWQ
The Water (Hydrologic) Cycle
Water is the basis of all living processes on Earth. When examining the stores of water on Earth, 97.5 percent of it is non-potable (unable to be safely consumed by living organisms) salt water (Figure 6). The remaining 2.5% of water is freshwater, but 99% of that freshwater is locked underground as water or as ice and inaccessible for use. Thus, less than 1 percent of freshwater is easily accessible from lakes and rivers. Many living things, such as plants, animals, and fungi, are dependent on that small amount of fresh surface water, a lack of which can have massive effects on ecosystem dynamics. To be successful, organisms must adapt to fluctuating water supplies. Humans, of course, have developed technologies to increase water availability, such as digging wells to harvest groundwater, storing rainwater, and using desalination to obtain drinkable water from the ocean.
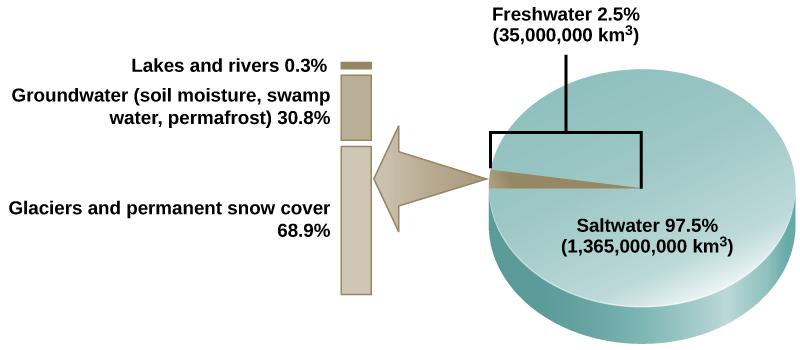
Figure 6. Only 2.5 percent of water on Earth is freshwater, and less than 1 percent of freshwater is easily accessible to living things. (Credit: This file is licensed under the Creative Commons Attribution 4.0 International license.)
Water cycling is extremely important to ecosystem dynamics. Water has a major influence on climate and, thus, on the environments of ecosystems. The water cycle (Figure 7) is driven by the sun’s energy as it warms the oceans and other surface waters. This leads to the conversion of liquid water on Earth’s surface to water vapor (gas) in the atmosphere (evaporation) and the conversion of ice to water vapor (sublimation), which deposits large amounts of water vapor into the atmosphere. Over time, this water vapor condenses into clouds as liquid or frozen droplets (condensation) and eventually returns to the Earth’s surface as precipitation in the form of rain or snow. Rain eventually permeates into the ground, where it may evaporate again if it is near the surface, flow beneath the surface, or be stored for long periods. In some cases, the rain and melted snow may become surface runoff, which delivers the water across the surface of the Earth to streams, rivers, lakes, and oceans. Rain and surface runoff are major ways in which minerals, including carbon, nitrogen, phosphorus, and sulfur, are transported from land to water. The environmental effects of runoff will be discussed later as these cycles are described.
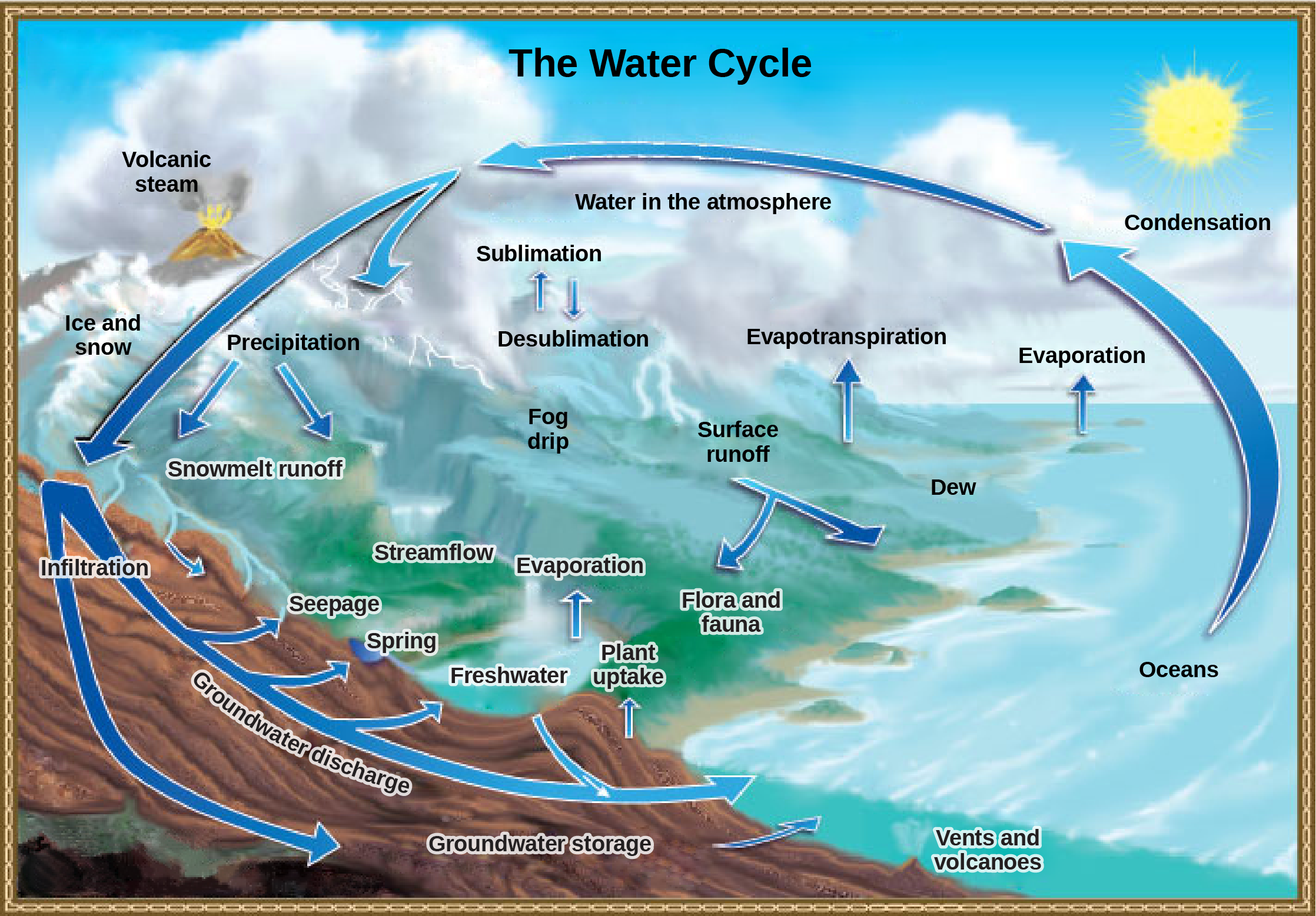
Figure 7. Water from the land and oceans enters the atmosphere by evaporation or sublimation, where it condenses into clouds and falls as rain or snow. Precipitated water may enter freshwater bodies or infiltrate the soil. The cycle is complete when surface or groundwater reenters the ocean. (Credit: modification of work by John M. Evans and Howard Perlman, USGS)
The Carbon Cycle
Carbon is the second most abundant element in living organisms, present in all organic molecules, and its role in the structure of organic molecules is of primary importance to living organisms. The carbon cycle is most easily studied as two interconnected sub-cycles: one dealing with rapid carbon exchange among living organisms and the other dealing with the long-term cycling of carbon through geologic processes. The entire carbon cycle is shown in Figure 8.
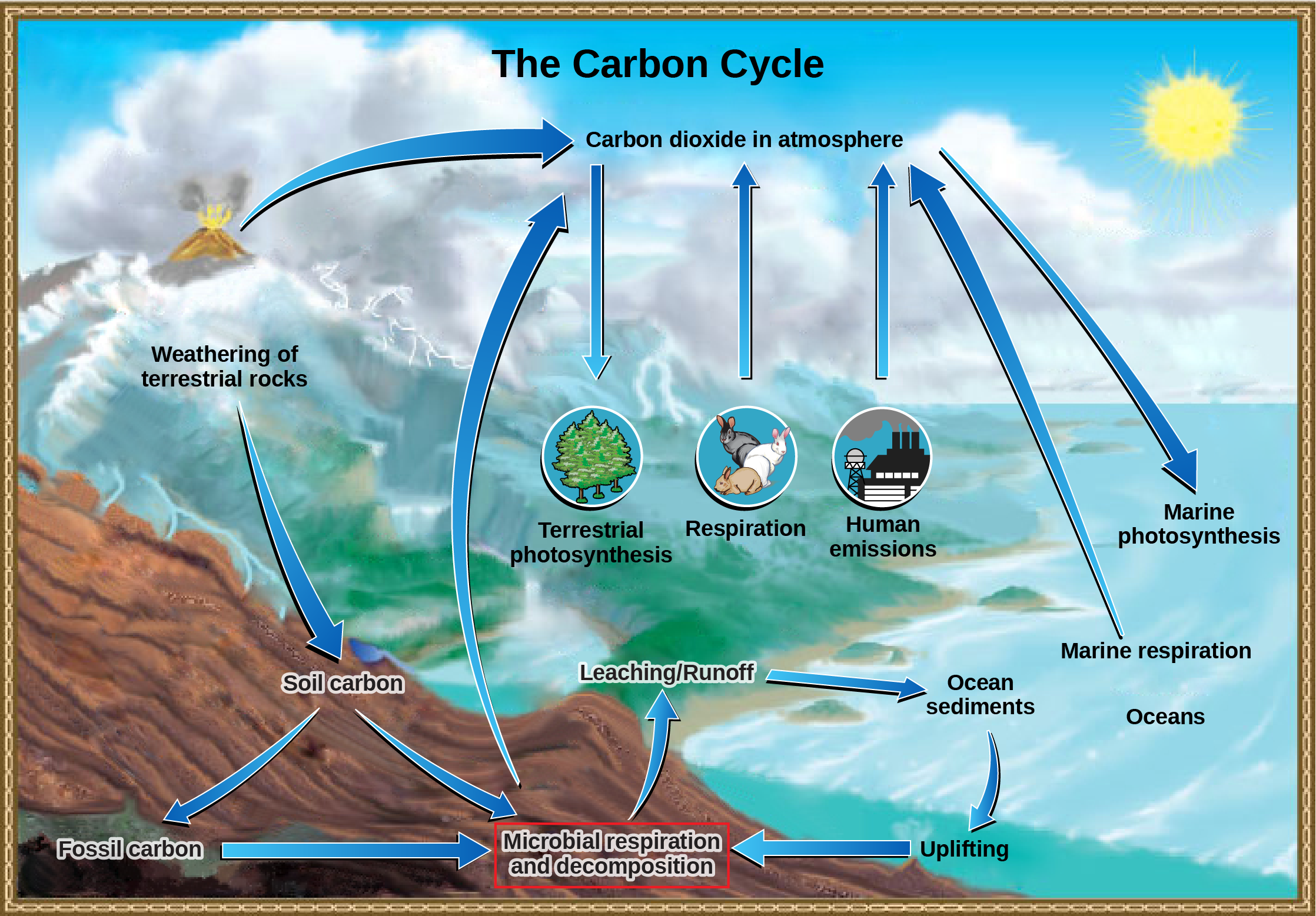
Figure 8. Carbon dioxide gas exists in the atmosphere and is dissolved in water. Photosynthesis converts carbon dioxide gas to organic carbon, and respiration cycles the organic carbon back into carbon dioxide gas. Long-term storage of organic carbon occurs when matter from living organisms is buried deep underground and becomes fossilized. Volcanic activity and, more recently, human emissions, bring this stored carbon back into the carbon cycle. (Credit: modification of work by John M. Evans and Howard Perlman, USGS)
The Biological Carbon Cycle
Heterotrophs and autotrophs regularly exchange carbon. Photoautotrophs use energy from the Sun and carbon dioxide to make carbon-based (organic) molecules and oxygen. Heterotrophs consume the high-energy carbon compounds produced by the autotrophs and break them down through a series of chemical reactions called respiration to make the molecule ATP, which cells can use for energy. The most efficient type of respiration, aerobic respiration, requires oxygen, which is also a byproduct of photosynthesis. During respiration, heterotrophs release carbon dioxide back into the atmosphere, which can then be used by autotrophs to perform photosynthesis again. The autotrophs can then use the carbon dioxide to conduct photosynthesis once again. Thus, there is a constant exchange of oxygen and carbon dioxide between autotrophs and heterotrophs.
The Biogeochemical Carbon Cycle
The movement of carbon through the land, water, and air is complex, and in many cases, it occurs much more slowly geologically than as seen between living organisms. Carbon is stored for long periods in what are known as carbon reservoirs. The atmosphere is a major reservoir of carbon in the form of carbon dioxide and is essential to the process of photosynthesis. The level of carbon dioxide in the atmosphere is greatly influenced by the reservoir of carbon in the oceans. Carbon dioxide (CO2) from the atmosphere dissolves in water and combines with water molecules to form carbonic acid, which is then converted into bicarbonate and carbonate ions. Some of these ions combine with calcium in seawater to form calcium carbonate (CaCO3), which is a major component of the shells of marine organisms. When these organisms die, their shells form sediments on the ocean floor. Over geologic time, the calcium carbonate joins together to form rocks, known as limestone, which comprises the largest carbon reservoir on Earth.
On land, carbon is stored in soil as a result of the decomposition of living organisms (by decomposers) or from weathering of terrestrial rock and minerals. This carbon can be leached into water reservoirs by surface runoff. Deeper underground, on land and at sea, are fossil fuels: the anaerobically decomposed remains of plants and animals that take millions of years to form. Carbon sediments from the ocean floor are taken deep within the Earth by the process of subduction: the movement of one tectonic plate beneath another. Carbon can enter the atmosphere geologically from land (including land beneath the surface of the ocean) by the eruption of volcanoes or from volcanic hydrothermal vents.
The problem of Global Warming that we are witnessing today is directly linked to the carbon cycle. Humans contribute to atmospheric carbon dioxide by burning fossil fuels for energy, raising livestock, and deforestation. Since the Industrial Revolution, which began in the 18th century, humans have significantly increased the release of carbon dioxide into the atmosphere by burning fossil fuels, thus releasing carbon that had been previously stored underground for millions of years. This excess carbon dioxide has caused the earth’s atmosphere to retain more heat than previously.
Animal husbandry by humans also increases atmospheric carbon dioxide. The large numbers of land animals raised to feed the Earth’s growing population results in increased carbon dioxide levels in the atmosphere due to farming practices, livestock respiration, and methane production. Humans also change the natural landscape for agriculture and to build communities in which to live. This deforestation reduces the number of autotrophs that are available to convert carbon dioxide from the air back into biomass. Although much of the debate about the future effects of increasing atmospheric carbon on climate change focuses on fossils fuels, scientists take natural processes, such as volcanoes and respiration, into account as they model and predict the future impact of this increase.
The Nitrogen Cycle
Getting nitrogen from the atmosphere in living organisms is difficult. Even though nitrogen gas (N2) comprises approximately 78 percent of the air in the atmosphere, plants and phytoplankton are not able to incorporate it into their biomass directly. Instead, atmospheric nitrogen must be converted into a form that can be used by living organisms through a process known as nitrogen fixation, during which inorganic nitrogen gas (N2) from the air is incorporated into organic compounds. Nitrogen fixation is carried out by free-living bacteria found in the environment and symbiotic bacteria that live in the root nodules of legumes (such as peas, beans, and peanuts). In fact, farmers and gardeners often grow peas both for their produce and to naturally add nitrogen to the soil. This practice goes back to ancient times, even if the science has only been recently understood.
Organic nitrogen is especially important to the study of ecosystem dynamics since many ecosystem processes, such as primary production and decomposition, are limited by the available supply of nitrogen. As shown in Figure 9, the nitrogen that enters living systems by nitrogen fixation is also converted from organic nitrogen back into nitrogen gas by bacteria. This process occurs in three steps in terrestrial systems. First, the ammonification process converts nitrogenous waste from living animals or from the remains of dead animals into ammonium (NH4+) by certain bacteria and fungi. Second, the ammonium is converted to nitrites (NO2−) by bacteria through a process known as nitrification. Subsequently, nitrites are converted to nitrates (NO3−) by similar organisms. Third, the process of denitrification occurs, whereby bacteria convert the nitrates into nitrogen gas, allowing it to reenter the atmosphere.
A similar process occurs in the marine nitrogen cycle, where the ammonification, nitrification, and denitrification processes are performed by marine bacteria. Some of this nitrogen falls to the ocean floor as sediment, which can then be moved to land in geologic time by uplift of the Earth’s surface and thereby incorporated into terrestrial rock. Although the movement of nitrogen from rock directly into living systems has been traditionally seen as insignificant compared with nitrogen fixed from the atmosphere, a recent study showed that this process may be more significant than previously thought and should be included in any study of the global nitrogen cycle.
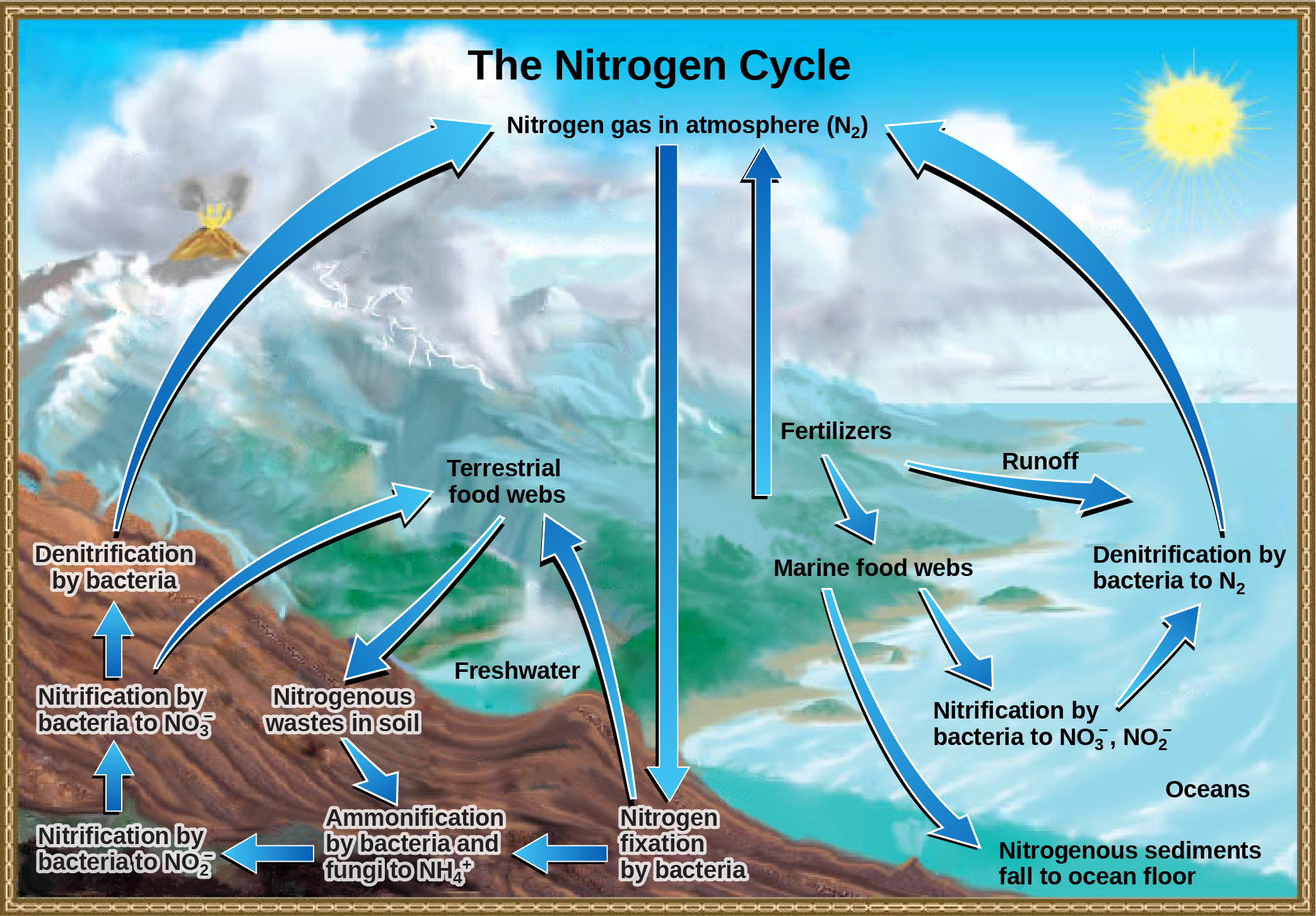
Figure 9. Nitrogen enters the living world from the atmosphere via nitrogen-fixing bacteria. This nitrogen and nitrogenous waste from animals is then processed back into gaseous nitrogen by soil bacteria, which also supply terrestrial food webs with the organic nitrogen they need. (Credit: modification of work by John M. Evans and Howard Perlman, USGS)
Human activity can alter the natural concentration of nitrogen in the atmosphere through the combustion of fossil fuels, which releases nitrogen oxides. Increased atmospheric nitrogen is associated with several negative effects on Earth’s ecosystems through the production of acid rain (as nitric acid, HNO3) and greenhouse gas (as nitrous oxide, N2O) potentially causing climate change.
The Phosphorus Cycle
Phosphorus is a major component of nucleic acid and phospholipids, and, as calcium phosphate, makes up the supportive components of our bones. Phosphorus is often the limiting nutrient (necessary for growth) in aquatic ecosystems (Figure 10). Phosphorus occurs in nature as the phosphate ion (PO43−). In addition to phosphate runoff as a result of human activity, natural surface runoff occurs when it is leached from phosphate-containing rock by weathering, thus sending phosphates into rivers, lakes, and the ocean. This rock has its origins in the ocean. Phosphate-containing ocean sediments form primarily from the bodies of ocean organisms and from their excretions. However, in remote regions, volcanic ash, aerosols, and mineral dust may also be significant phosphate sources. This sediment then is moved to land over geologic time by the uplifting of areas of the Earth’s surface.
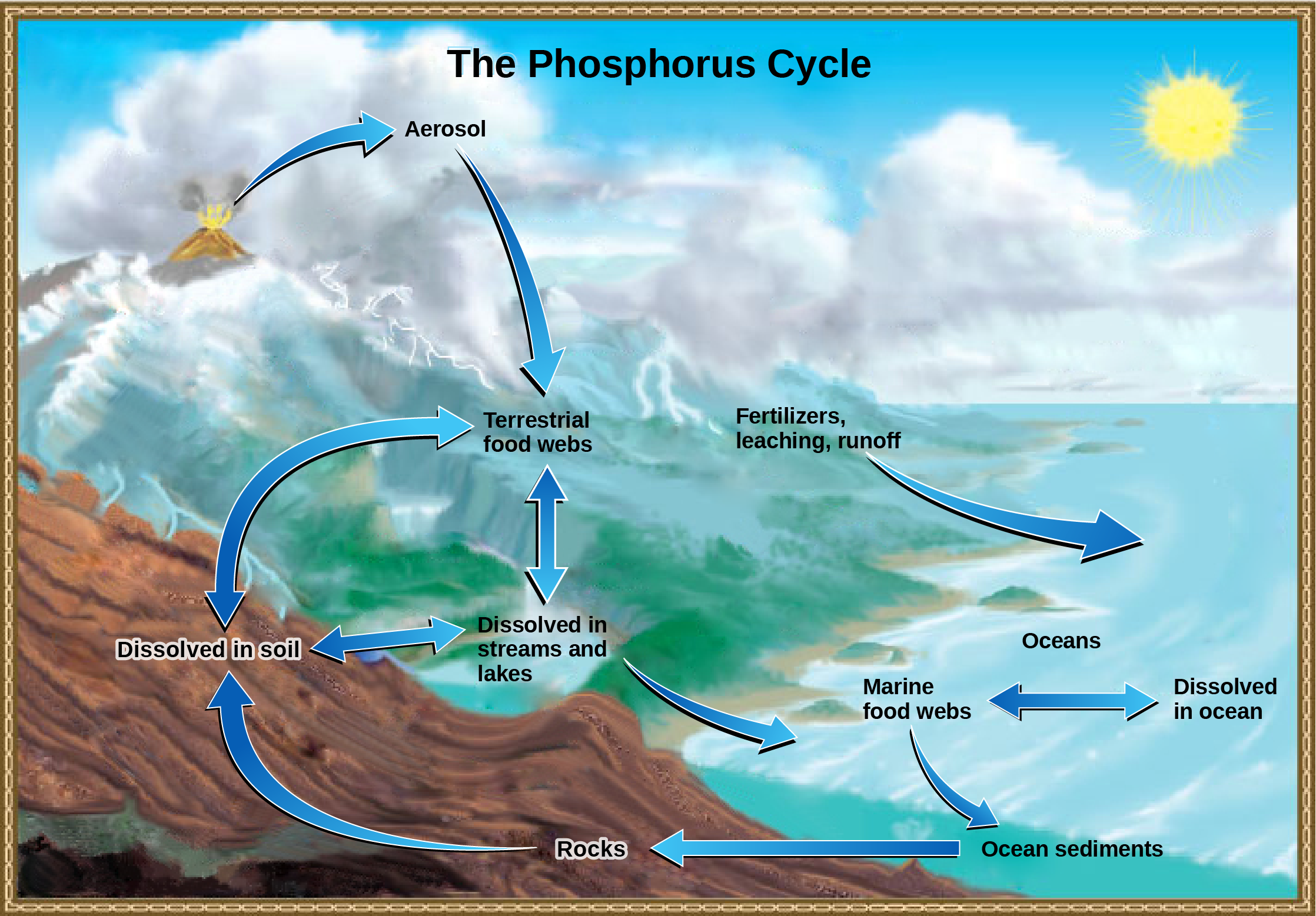
Figure 10. In nature, phosphorus exists as the phosphate ion (PO43−). Weathering of rocks and volcanic activity releases phosphate into the soil, water, and air, where it becomes available to terrestrial food webs. Phosphate enters the oceans via surface runoff, groundwater flow, and river flow. Phosphate dissolved in ocean water cycles into marine food webs. Some phosphate from the marine food webs falls to the ocean floor, where it forms sediment. (Credit: modification of work by John M. Evans and Howard Perlman, USGS)
Phosphorus is also reciprocally exchanged between phosphate dissolved in the ocean and marine ecosystems. The movement of phosphate from the ocean to the land and through the soil is extremely slow, with the average phosphate ion having an oceanic residence time between 20,000 and 100,000 years.
Eutrophication and Dead Zones
Excess phosphorus and nitrogen enters aquatic ecosystems from fertilizer runoff, sewage, and the use of phosphate in detergents, all of which are caused by human activity. Phosphorus and nitrogen are limiting factors in aquatic ecosystems. Thus, an influx of these nutrients can lead to the overgrowth of organisms, which can deplete dissolved oxygen, causing eutrophication. Eutrophication leads to the death of many aquatic flora and fauna, resulting in dead zones in lakes and at the mouths of many major rivers.
A dead zone is an area within a freshwater or marine ecosystem where large areas are depleted of their normal flora and fauna; these zones can be caused by eutrophication, oil spills, dumping of toxic chemicals, and other human activities. The number of dead zones has been increasing for several years, and more than 400 of these zones were present as of 2018 (Figure 11). One of the worst dead zones is off the coast of the United States in the Gulf of Mexico, where fertilizer runoff from the Mississippi River basin has created a dead zone of over 8463 square miles. Phosphate and nitrate runoff from fertilizers also negatively affect several lake and bay ecosystems including the Chesapeake Bay in the eastern United States.
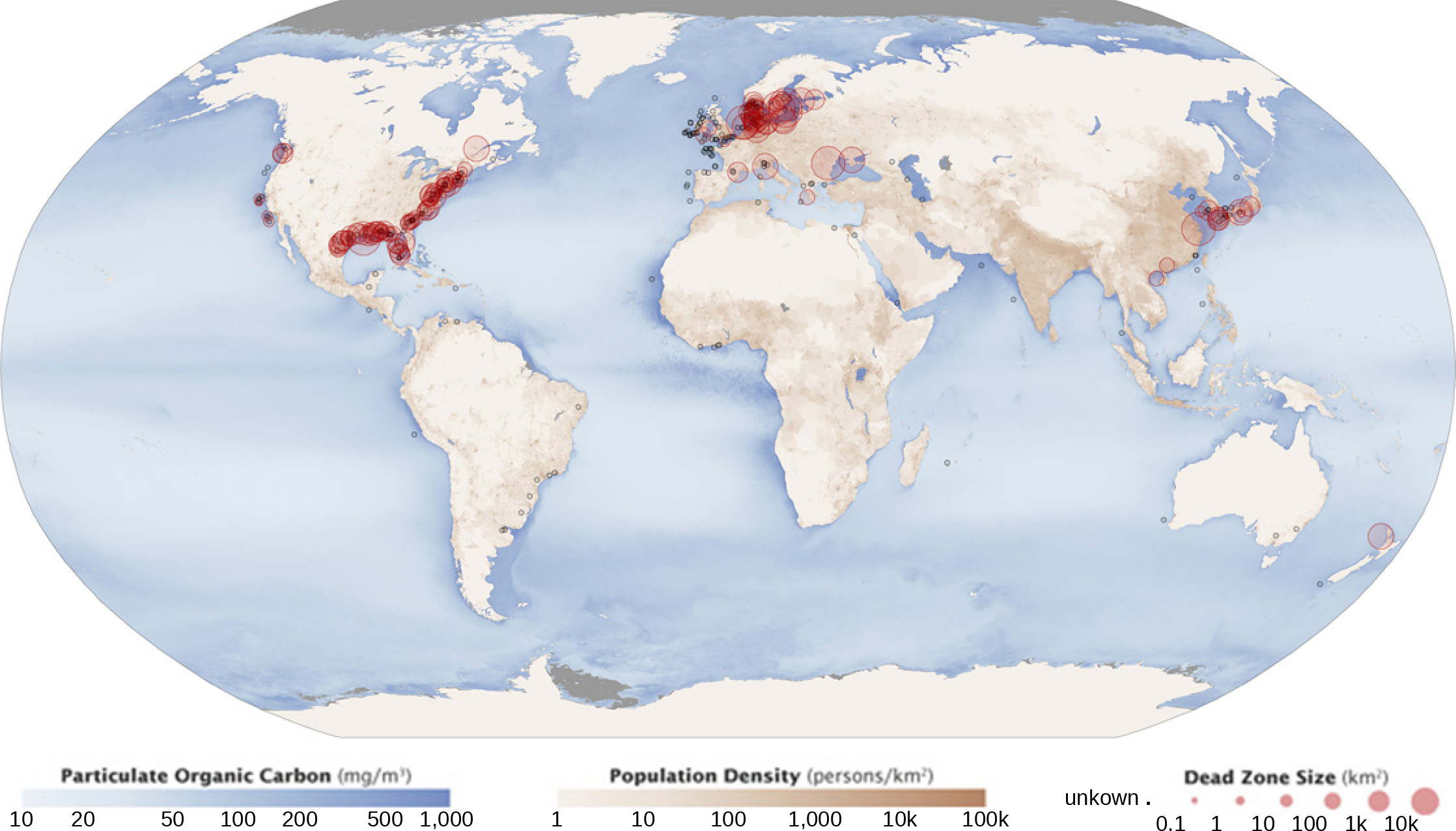
Figure 11. Dead zones occur when phosphorus and nitrogen from fertilizers cause excessive growth of microorganisms, which depletes oxygen and kills fauna. Worldwide, large dead zones are found in coastal areas of high population density. (Credit: NASA Earth Observatory)
3.6 Case Study: Eutrophication in Chesapeake Bay
The Chesapeake Bay has long been valued as one of the most scenic areas on Earth; it is now in distress and is recognized as a declining ecosystem. In the 1970s, the Chesapeake Bay was one of the first ecosystems to have identified dead zones, which continue to kill many fish and bottom-dwelling species, such as clams, oysters, and worms. Several species have declined in the Chesapeake Bay due to surface water runoff containing excess nutrients from artificial fertilizer used primarily on farm fields. But the source of the fertilizers (with high nitrogen and phosphate content) is not limited to agricultural practices. There are many nearby urban areas that contribute nitrogen and phosphorus to the more than 150 rivers and streams that empty into the bay. These streams carry fertilizer runoff from lawns and gardens. Thus, the decline of the Chesapeake Bay is a complex issue and requires the cooperation of industry, agriculture, and everyday homeowners (Figure 12).

Figure 12. This (a) satellite image shows the Chesapeake Bay, an ecosystem affected by phosphate and nitrate runoff. A (b) member of the Army Corps of Engineers holds a clump of oysters being used as a part of the oyster restoration effort in the bay. (Credit a: modification of work by NASA/MODIS; credit b: modification of work by U.S. Army)
Of particular interest to conservationists is the oyster population; it is estimated that more than 200,000 acres of oyster reefs existed in the bay in the 1700s, but that number has now declined to only 36,000 acres. Oyster harvesting was once a major industry for Chesapeake Bay, but it declined 88 percent between 1982 and 2007. This decline was due not only to fertilizer runoff and dead zones but also to overharvesting. Oysters require a certain minimum population density because they must be in close proximity to reproduce. Human activity has altered the oyster population and locations, greatly disrupting the ecosystem.
The restoration of the oyster population in the Chesapeake Bay has been ongoing for several years with mixed success. Not only are oysters edible, but they also clean up the bay. Oysters are filter feeders, and as they eat, they clean the water around them. In the 1700s, it was estimated that it took only a few days for the oyster population to filter the entire volume of the bay. Today, with changed water conditions, it is estimated that the present population would take nearly a year to do the same job.
Restoration efforts have been ongoing for several years by nonprofit organizations, such as the Chesapeake Bay Foundation. The restoration goal is to find a way to increase population density so the oysters can reproduce more efficiently. Many disease-resistant varieties (developed at the Virginia Institute of Marine Science for the College of William and Mary) are now available and have been used in the construction of experimental oyster reefs. Efforts to clean and restore the bay by Virginia and Delaware have been hampered because much of the pollution entering the bay comes from other states, which stresses the need for interstate cooperation to gain successful restoration.
The new, hearty oyster strains have also spawned a new and economically viable industry—oyster aquaculture—which not only supplies oysters for food and profit, but also has the added benefit of cleaning the bay.
The following video gives an in-depth review of issues related to the health of the Chesapeake Bay ecosystem:
NASA eClips. (2010, March 18). Real World: NASA and the Chesapeake Bay [Video – YouTube] https://youtu.be/BwKU3FVoGMk
Attributions:
Content in this chapter includes original work created by Lauren Roberts and Paul Bosch as well as from the following sources, with some modifications:
Biology by OpenStax is licensed under CC BY 3.0. Modified from the original by Matthew R. Fisher.
Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0. Modified from the original by Matthew R. Fisher.

