Chapter 2 Biological Organization
Chapter 2 Outline
2.1 Levels of Biological Organization
2.2 Matter: Atoms, Molecules, and Compounds
2.4 Acids and Bases, pH and Buffers
2.6 Cell Theory and Types of Cells
Learning Outcomes
After studying this chapter, each student should be able to:
- 2.1 Describe the various levels of biological organization, from atoms to organisms, and species to biosphere
- 2.2 Understand how atoms can make up molecules and compounds, and how they may be bonded
- 2.3 Be able to describe the major properties of water, especially as water relates to living things
- 2.4 Describe how acids differ from bases, what pH measures, and the importance of buffers in living systems
- 2.5 Be able to recognize and describe the major organic molecules, such as carbohydrates, lipids, proteins, and nucleic acids
- 2.6 Describe the cell theory, and be able to differentiate between prokaryotes & eukaryotes, animal & plant cells, and the various cellular organelles
2.1 Levels of Biological Organization
Environmental science is the interdisciplinary study of the interaction of living and non-living parts of the environment, with special focus on the impact of humans on the environment. Environmental biology more specifically focuses on studying living (biotic) organisms and the non-living (abiotic) aspects of the environment with which they interact. To understand life, you first have to understand what living organisms are made of and how scientists define “life”.
Living things are highly organized and structured, following a hierarchy of scale from small to large (Figure 1). The atom is the smallest and most fundamental unit of matter. Atoms (such as hydrogen, oxygen, and carbon) combine to form molecules which are chemical structures consisting of at least two atoms held together by a chemical bond. Examples of molecules include water (H2O), oxygen gas (O2), and carbon dioxide (CO2), as well as sugars, fats, proteins, and nucleic acids. In plants, animals, and many other types of organisms, molecules come together in specific ways to create structures called organelles.
Organelles (such as a nucleus or mitochondria) are small structures that exist within cells and perform specialized functions. All living things are made of one or more cells.
A bacterium is a single-celled living organism. Other organisms, like plants and animals, are made up of multiple cells working together, which each cell carrying out a specific function. For example, humans have red blood cells that carry oxygen around the body and nerve cells (neurons) that transmit information from one area of the body to another. Plants have cells that make up vascular tissue that allows the organism to transport water and nutrients. Other cells contain specialized organelles to allow them to perform an important chemical process, known as photosynthesis.
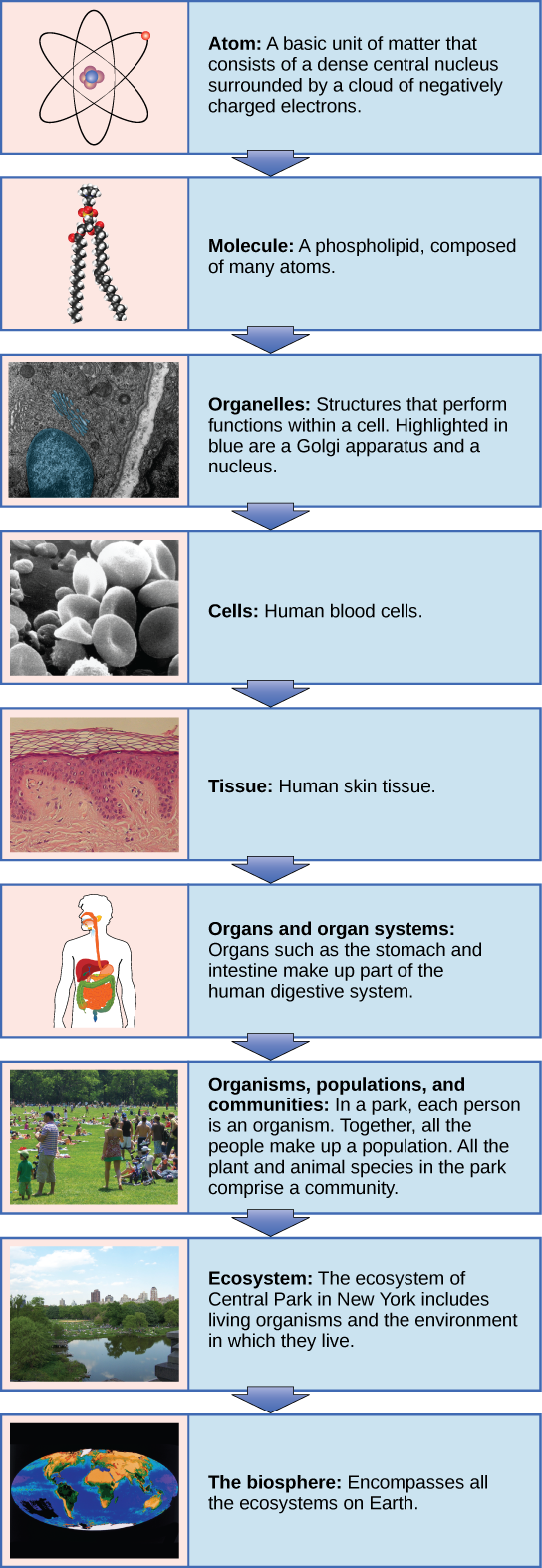
Figure 1. From an atom to the entire Earth, biology examines all aspects of life. (Credit: “Molecule”: modification of work by Jane Whitney; (Credits: “Organelles“: modification of work by Louisa Howard; “Cells“: modification of work by Bruce Wetzel, Harry Schaefer, National Cancer Institute; “Tissue“: modification of work by “Kilbad”/Wikimedia Commons; “Organs“: modification of work by Mariana Ruiz Villareal, Joaquim Alves Gaspar; “Organisms“: modification of work by Peter Dutton; “Ecosystem“: modification of work by “gigi4791″/Flickr; “Biosphere“: modification of work by NASA)
In most multicellular organisms, cells combine to make tissues (such as cardiac muscle tissue in some animals and vascular tissue in some plants), which are groups of similar cells carrying out the same function. Organs (such as your heart, lungs, and kidneys) are collections of tissues grouped together based on a common function. Organs are present not only in animals but also in plants.
An organ system is a higher level of organization that consists of functionally related organs. For example, vertebrate animals have many organ systems, such as the cardiovascular system that transports blood throughout the body and to and from the lungs; it includes organs such as the heart and blood vessels. An organism is an individual living entity. For example, each tree in a forest or a single mushroom is an organism.
Each organism is classified as belonging to a particular species, which is anatomically, physiologically and reproductively distinct from other species. So a maple tree in the forest might be a sugar maple (Acer saccharum), distinct from the oaks and beech trees, and also other species of maples. All the individuals of a species living within a specific area are collectively called a population, so all of the sugar maples in a defined forest area make up a population. A community consists of different populations inhabiting a common area. For instance, all of the various species of trees, flowers, insects, mushrooms, squirrels, and other populations in a forest form the forest’s community. The forest itself is an ecosystem. An ecosystem consists of all the living (biotic) things in a particular area together with the non-living (abiotic) factors of that environment, such as the soil or rainwater. The highest level of organization, the biosphere, is the collection of all ecosystems, and represents all of the zones of life on Earth. The biosphere includes all life on land, in water, and where living things exist in the atmosphere.
Ecology is the branch of biology that studies the relationships between organisms and their biotic and abiotic environment. Ecology may focus on studying particular species, or the populations, communities, ecosystems, or biosphere to which they belong. Environmental Biology, more than other branches of biology, focuses primarily on ecology and ecosystems.
2.2 Matter: Atoms, Molecules, and Compounds
At its most fundamental level, life is made of matter. Matter is something that occupies space and has mass. All matter is composed of elements, substances that cannot be broken down or transformed chemically into other substances. A total of 118 elements have been defined; however, only 92 occur naturally and fewer than 30 are found in living cells. Each element is designated by its chemical symbol (such as H, N, O, C, and Na), and possesses unique properties. These unique properties allow elements to combine and to bond with each other in specific ways.
An atom is the smallest unit of an element that retains all of the chemical properties of that element. All matter, whether it is a rock or an organism, is made of atoms. All atoms contain subatomic particles which include protons, electrons, and neutrons (Figure 2). The only exception is hydrogen (H), which is made of one proton and one electron, but does not have any neutrons.
A proton is a positively charged particle that resides in the nucleus (the core) of an atom and has a mass of 1 atomic mass unit (amu) and a charge of +1. An electron is a negatively charged particle that travels in the space around the nucleus, called the orbitals or shells. It has a negligible mass (0 amu) and has a charge of –1. Neutrons, like protons, reside in the nucleus of an atom. Also like protons, they have a mass of 1 amu, but they do not have a charge. The positive (protons) and negative (electrons) charges balance each other in a neutral atom, which has a net zero charge.
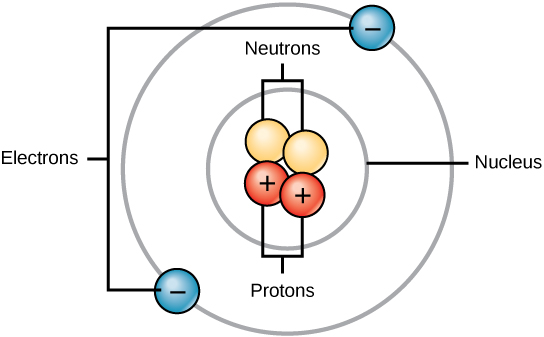
Figure 2. Atoms are made up of protons and neutrons located within the nucleus, and electrons surrounding the nucleus. (Credit: “Atoms” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
Each element contains a different number of protons. The atomic number of an element is equal to the number of protons that element contains. The Periodic Table of the Elements, on the wall of many science classrooms, is based on the atomic numbers. The mass number is the number of protons plus the number of neutrons of that element. It is possible to determine the number of neutrons in an atom of an element by subtracting the atomic number from the mass number (Figure 3).
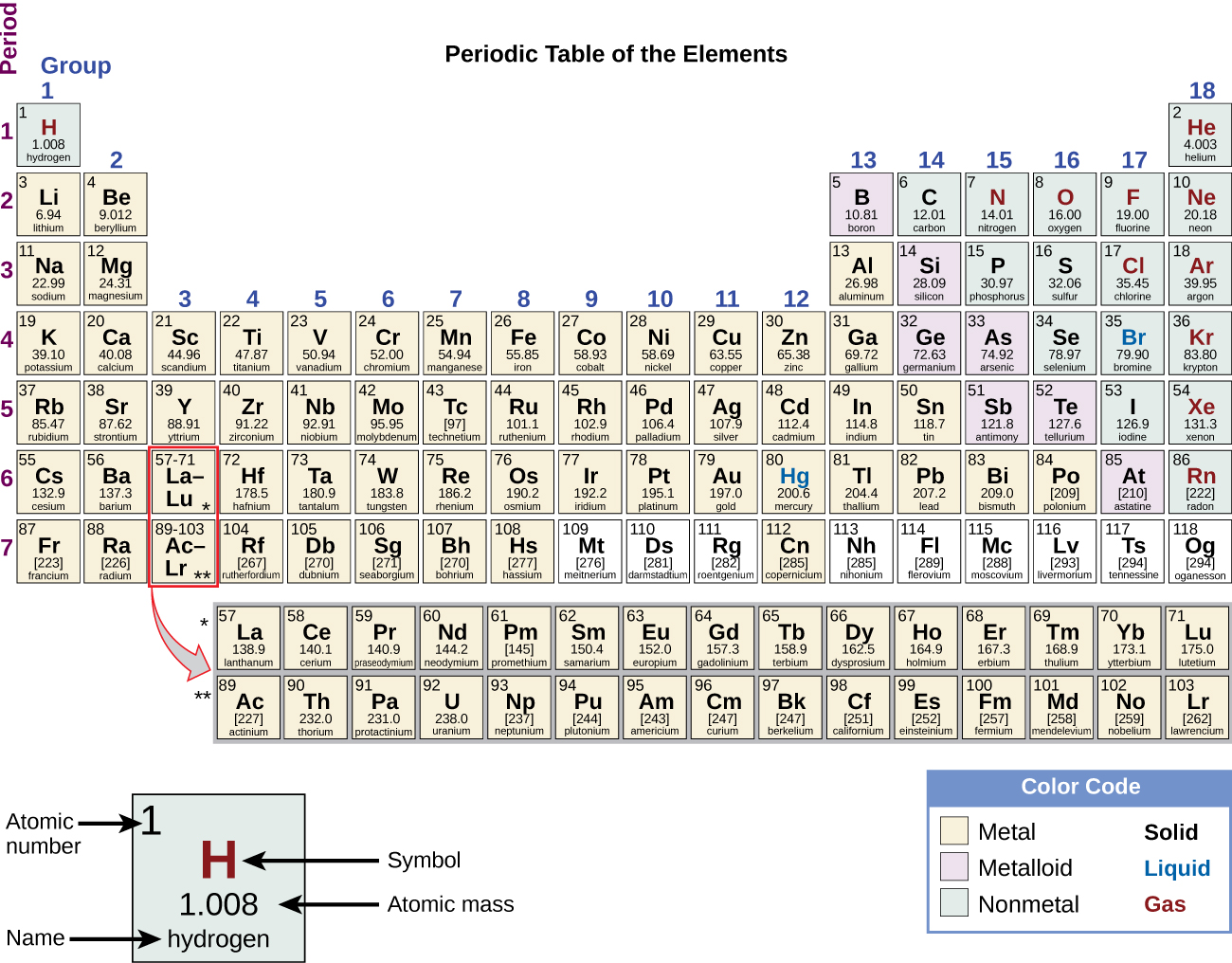
Figure 3 Arranged in columns and rows based on the characteristics of the elements, the periodic table provides key information about the elements and how they might interact with each other to form molecules. Most periodic tables provide a key or legend to the information they contain. (Credit: “Periodic Table” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
Although all atoms of a particular element must have the same number of protons, the number of neutrons in each atom may be different. Isotopes are different forms of the same element that have the same number of protons, but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes. Carbon 12, the most common isotope of carbon, contains six protons and six neutrons. Therefore, it has a mass number of 12 (6+6) and an atomic number of 6 (which makes it carbon). Carbon 14 contains six protons and eight neutrons. Therefore, it has a mass number of 14 (6+8) (Figure 4). Even though the mass number is different from carbon 12, carbon 14 still has an atomic number of 6, meaning it is still the element carbon. These two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more stable elements. These are called radioactive isotopes or radioisotopes.
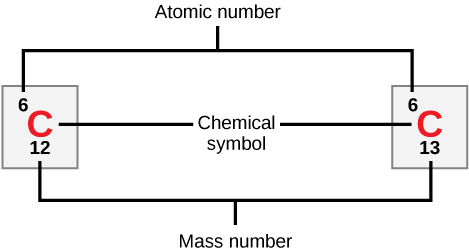
Figure 4. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Its relative atomic mass is 12.011. (Credit: “Carbon Isotopes” by OpenStax Biology, 2nd ed. is licensed under CC BY 4.0)
Chemical Bonds
Often, atoms combine to form molecules. A molecule is a chemical made from two or more atoms bonded together. Some molecules are very simple, like water (H2O) (Figure 5), which consists of just one oxygen atom and two hydrogen atoms bonded together. Other molecules, such as DNA, are made of millions of atoms.
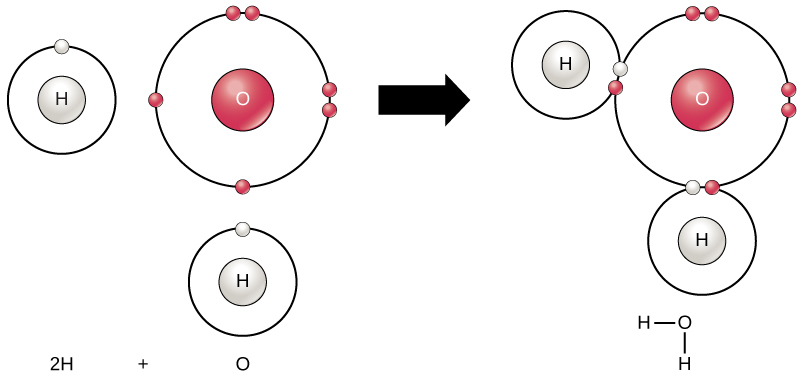
Figure 5. Two or more atoms may bond with each other to form a molecule. When two hydrogens and an oxygen share electrons via covalent bonds it forms a water molecule. (Credit: “Water Molecule” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
How elements interact with one another depends on the number of electrons an atom has and how they are arranged. When an atom does not contain equal numbers of protons and electrons it is called an ion. Because the number of electrons does not equal the number of protons, each ion has a net charge. For example, if sodium loses an electron, it now has 11 protons and 10 electrons, leaving it with an overall charge of +1. Positive ions are formed by losing electrons and are called cations. Negative ions are formed by gaining electrons and are called anions (Figure 6). Elemental anionic names are changed to end in -ide. As an example, when chlorine becomes an ion it is referred to as chloride.
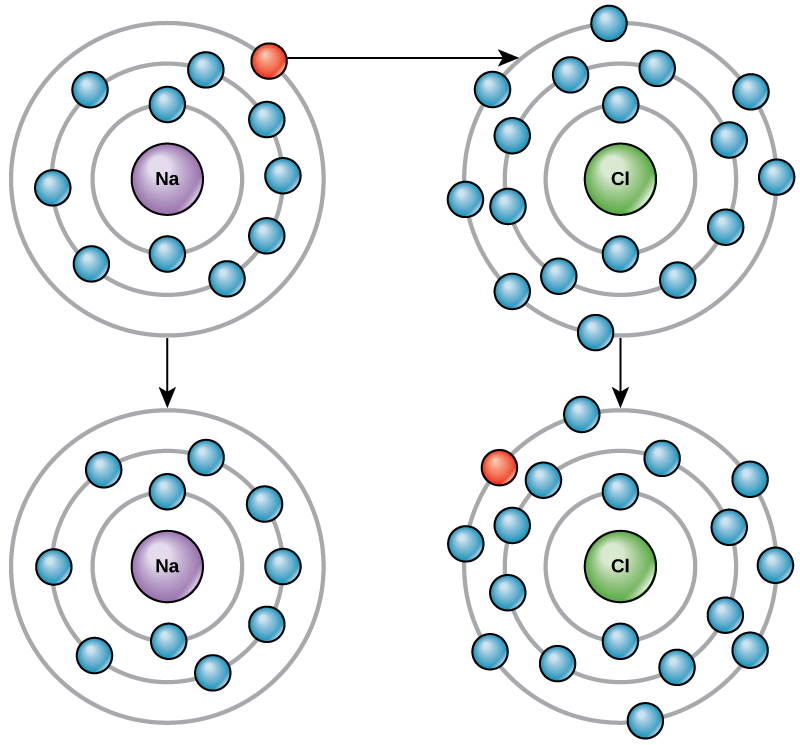
Figure 6. Elements tend to fill their outermost shells with electrons. To do this, they can either donate or accept electrons from other elements. (Credit: “Ion Formation” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
Ionic and covalent bonds are strong bonds formed between two atoms. These bonds hold atoms together in a relatively stable state. Ionic bonds are formed between two oppositely charged ions, like Na+ and Cl–(a cation and an anion). Because positive and negative charges attract, these ions are held together much like two oppositely charged magnets would stick together. Covalent bonds form when electrons are shared between two atoms. Each atom shares one of their electrons, which then orbits the nuclei of both atoms, holding the two atoms together. Covalent bonds are the strongest and most common form of chemical bond in organisms.
Covalent bonds come in two varieties: polar and nonpolar (Figure 7). A nonpolar covalent bond occurs when electrons are shared equally between the two atoms. Polar covalent bonds form when the electrons are shared unequally. The oxygen and hydrogen atoms in a molecule of water are held together by polar covalent bonds, making water a polar molecule. This gives water special properties that allows it to support life.
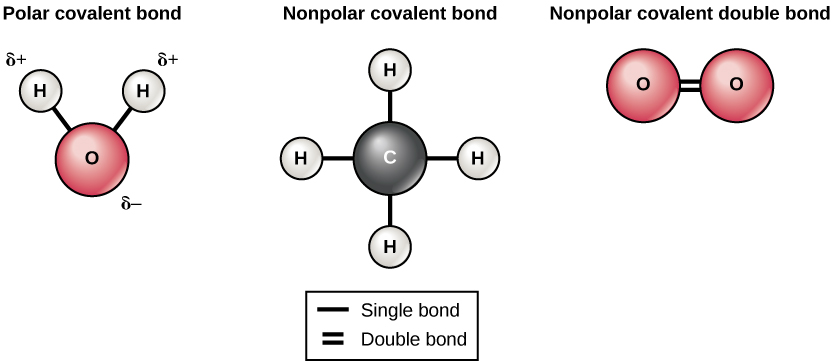
Figure 7. The water molecule (left) depicts a polar bond with a slightly positive charge on the hydrogen atoms and a slightly negative charge on the oxygen. Examples of nonpolar bonds include methane (middle) and oxygen (right). (Credit: “Covalent Bonds” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
When oxygen and hydrogen bond, for example, the shared electrons are pulled more strongly toward oxygen and thus farther away from hydrogen’s nucleus. Because the electrons move farther away from hydrogen, it becomes slightly positively charged (δ+). The oxygen becomes slightly negatively charged as the electrons become closer to it (δ–). If two molecules with polar covalent bonds approach one another, they can interact due to the attraction of opposite electrical charges. For example, the slight positive charge of hydrogen in a water molecule can be attracted to the slight negative charge of oxygen in a different water molecule (Figure 8). This interaction between two polar molecules is called a hydrogen bond. This type of bond is very common in organisms. Notably, hydrogen bonds give water unique properties that sustain life.
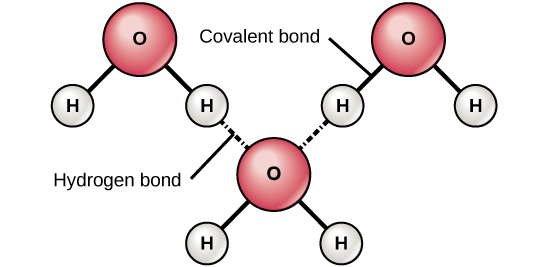
Figure 8. Hydrogen bonds form between slightly positive (δ+) and slightly negative (δ–) charges of polar covalent molecules, such as water. (Credit: “Hydrogen Bonds” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
2.3 Properties of Water
Do you ever wonder why scientists spend time looking for water on other planets? Water is one of the more abundant molecules in living cells and the one most critical to life as we know it. Approximately 60-70 percent of your body is made up of water. Without it, life simply would not exist.
Water is a polar molecule. The hydrogen and oxygen atoms within water molecules form polar covalent bonds. The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. There is no overall charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom. Water’s polarity gives it its unique properties that allow it to sustain life.
Each water molecule attracts other water molecules because of the slight positive and negative charges in the different parts of the molecule. Water also attracts other polar molecules (such as sugars). These polar molecules can dissolve in water and are referred to as hydrophilic (“water-loving”). Nonpolar molecules do not readily interact with water and are called hydrophobic (“water-fearing”). Oil is an example of a hydrophobic molecule (Figure 9).

Figure 9. As this macroscopic image of oil and water show, oil is a nonpolar compound and, hence, will not dissolve in water. Oil and water do not mix. (Credit: Gautam Dogra)
Water stabilizes temperature. The hydrogen bonds in water allow it to absorb and release heat energy more slowly than many other substances. This means that water moderates temperature changes within organisms and in their environments, because its temperature changes slowly despite large changes in the amount of energy it absorbs. When a large amount of energy is absorbed by water, the hydrogen bonds between the water molecules do eventually break, resulting in evaporation, which is the release of individual water molecules into the air. This evaporation has a cooling effect, because breaking hydrogen bonds requires an input of energy and takes heat away from the organism. Sweating is an example of evaporation that cools the human body.
Conversely, as temperatures drop, less energy is present to break the hydrogen bonds between water molecules. These bonds remain intact and begin to form a rigid, lattice-like structure (e.g., ice) (Figure 10a). When frozen, ice is less dense than liquid water (the molecules are farther apart). This means that ice floats on the surface of a body of water (Figure 10b). In lakes, ponds, and oceans, ice will form on the surface of the water, creating an insulating barrier to protect the animal and plant life beneath from freezing in the water. If this did not happen, plants and animals living in water would freeze in a block of ice and could not move freely, making life in cold temperatures difficult or impossible.
Water’s ability to absorb and release heat more slowly than other molecules also helps modulate temperatures within organisms and throughout the entire planet. Without this property, temperatures on Earth and in individual organisms would vary more greatly throughout the day and throughout the year, which would likely make conditions uninhabitable for life.
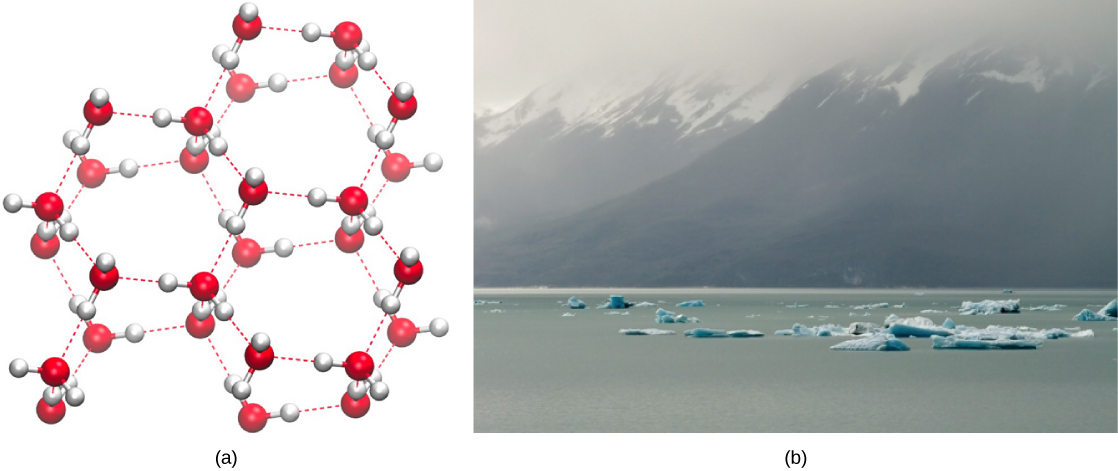
Figure 10. (a) The lattice structure of ice makes it less dense than the freely flowing molecules of liquid water. Ice’s lower density enables it to (b) float on water. (Credit a: modification of work by Jane Whitney; Credit b: modification of work by Carlos Ponte)
Water is also an excellent solvent. A solvent is a substance capable of dissolving another substance. Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. The substance dissolved is called a solute (such as salt or sugar). Together a solvent and solute make up a solution. The charged particles form hydrogen bonds with a surrounding layer of water molecules, as shown in Figure 11.
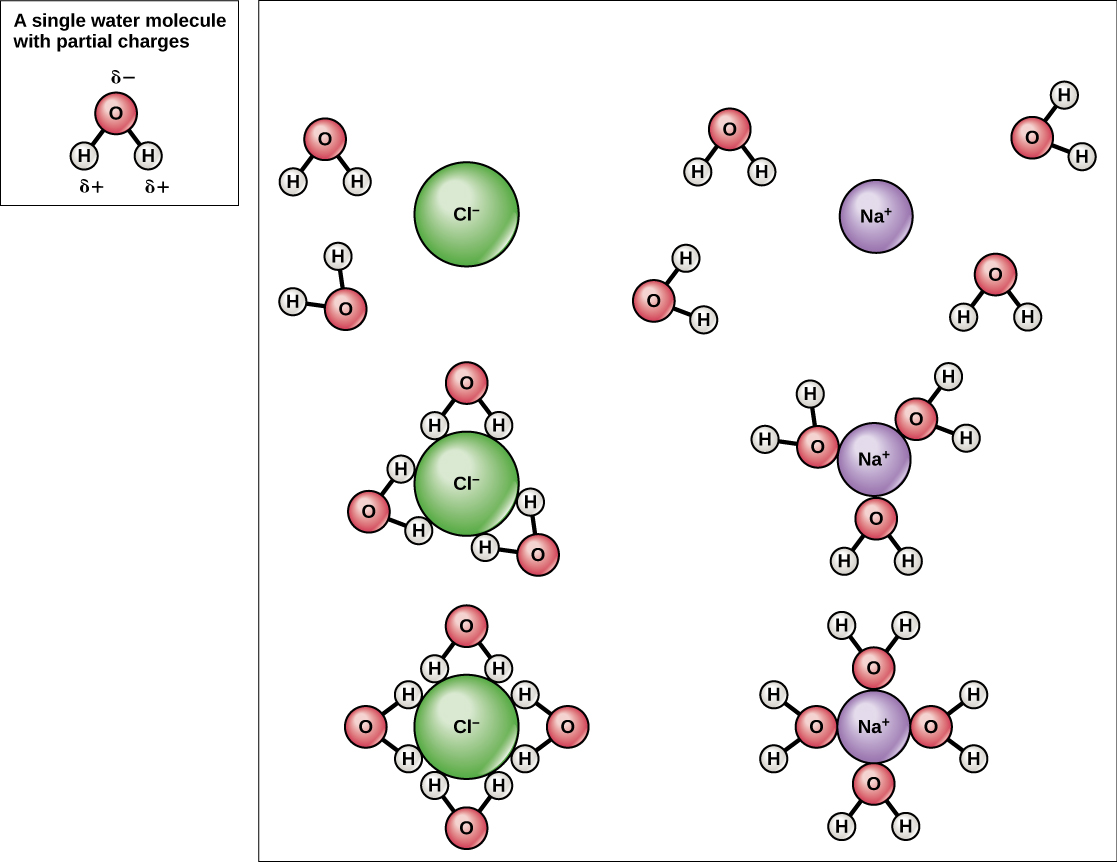
Figure 11. When table salt (NaCl) is mixed in water, spheres of hydration form around the ions. (Credit: “Water as a Solvent” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
Water is cohesive. Have you ever filled up a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water actually forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, the same kinds of molecules are attracted to each other. Water molecules, for example, are attracted to each other (because of hydrogen bonding).
Cohesion gives rise to surface tension (Figure 12), the capacity of a substance to withstand rupture when placed under tension or stress. When you drop a small scrap of paper onto a droplet of water, the paper floats on top of the water droplet, although the object is denser (heavier) than the water. This occurs because of the surface tension that is created by the hydrogen bonds between water molecules. Cohesion and surface tension keep the water molecules intact and the item floating on the top.

Figure 12. Water’s cohesive and adhesive properties allow this water strider (Gerris sp.) to stay afloat. Notice the indentations in the water where the insect’s feet put stress on the hydrogen bonds between water molecules. (Credit: Tim Vickers)
These cohesive forces are also related to the water’s property of adhesion (Figure 13), or the attraction between water molecules and other molecules. This is observed when water “climbs” up a straw placed in a glass of water. You will notice that the water appears to be higher on the sides of the straw than in the middle. This is because the water molecules are attracted to the straw and therefore adhere to it. Cohesive and adhesive forces are important for sustaining life. For example, these forces allow water to flow up from the roots to the top of a plant.
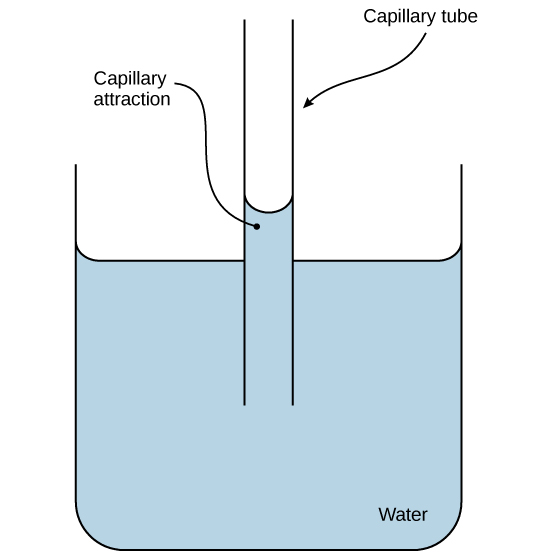
Figure 13. The adhesive forces exerted by the glass’ internal surface exceeding the cohesive forces between the water molecules themselves causes capillary action in a glass tube. (Credit: modification of work by Pearson-Scott Foresman, donated to the Wikimedia Foundation)
2.4 Acids and Bases, pH and Buffers
Acidity is another important characteristic of water. The pH of a solution is a measure of its acidity or alkalinity, which is related to the concentration of hydrogen ions (H+) in the solution. The pH scale ranges from 0 to 14 (Figure 14). A change of one unit on the pH scale represents a change in the concentration of hydrogen ions (H+) by a factor of 10; a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic (alkaline) and has a pH of 7.0. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is basic. The further away a substance is from a pH of 7.0, the stronger its acidity or alkalinity is. The blood in your veins is slightly alkaline (pH = 7.4). The environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic (pH = 9.0).

Figure 14. The pH scale measures the amount of hydrogen ions (H+) in a substance. (Credit: modification of work by Edward Stevens)
Acids are substances that increase the concentration of hydrogen ions (H+) in a solution and they lower pH, meaning they lower the number on the pH scale. Bases decrease the concentration of hydrogen ions (H+) in a solution while increasing the concentration of hydroxide ions (OH–) and the pH increases (i.e. the number gets higher). The stronger the acid, the more readily it donates H+. For example, hydrochloric acid and lemon juice are very acidic and readily give up H+ when added to water.
Conversely, bases are those substances that readily donate OH–. The hydroxide (OH–) ions combine with H+ to produce water, which decreases the concentration of free H+ in a solution and raises a substance’s pH. Sodium hydroxide (NaOH) and many household cleaners are very alkaline and give up OH– rapidly when placed in water, thereby raising the pH.
How is it that we can ingest or inhale acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H+ or OH–, keeping the pH of the body carefully maintained in a narrow range of acceptable values.
2.5 Major Organic Molecules
In addition to water, organic molecules are necessary for life to exist. Organic molecules are those that contain carbon covalently bonded to hydrogen. In addition, they may also contain oxygen, nitrogen, phosphorus, sulfur, and additional elements. It is often said that life is “carbon-based.” This means that carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many of the molecules found uniquely in living things. Other elements play important roles in biological molecules, but carbon certainly qualifies as the “foundation” element for molecules in living things. It is the bonding properties of carbon atoms that are responsible for its important role. There are four major classes of organic molecules: carbohydrates, lipids, proteins, and nucleic acids. Each is an important component of the cell and performs a wide array of functions.
Carbohydrates include both simple sugars, like glucose and fructose, and complex carbohydrates such as starch and cellulose. While many types of carbohydrates are used for energy, some are structural. For example, cellulose is a complex carbohydrate that adds rigidity and strength to the cell walls of plants. The suffix “-ose” denotes a carbohydrate, but note that not all carbohydrates follow this naming convention (e.g., starch).
Lipids include fats and other hydrophobic (“water-fearing”) organic molecules – a diverse group of compounds that are all insoluble in water, because they are nonpolar molecules (molecules that contain nonpolar covalent bonds). Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of lipids called fats. Lipids also provide insulation from the environment for plants and animals. For example, they help keep aquatic birds and mammals dry because of their water-repelling nature (Figure 15). Lipids are also the building blocks of many hormones and are an important constituent of cellular membranes. Lipids include fats, oils, waxes, phospholipids, and steroids.

Figure 15. Hydrophobic lipids in the fur of aquatic mammals, such as this river otter, protect them from the elements. (Credit: Ken Bosma)
Proteins, made up of subunits called amino acids, are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. The functions of proteins are very diverse because there are 20 different chemically distinct amino acids that form long chains, and the amino acids can be in any order. Proteins can function as enzymes, hormones, contractile fibers, cytoskeleton rods, and much more. Enzymes are vital to life because they act as catalysts in biochemical reactions (like digestion). A catalyst is a substance that speeds up a chemical reaction. Each enzyme is specific for the substrate (a reactant that binds to an enzyme) upon which it acts. Enzymes can function to break molecular bonds, to rearrange bonds, or to form new bonds.
Nucleic acids are very large molecules that are important genetically and allow for the continuity of life. They carry the genetic blueprint of a cell and thus the instructions for its functionality. The two main types of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is the genetic material found in all organisms (Figure 16), ranging from single-celled bacteria to multicellular mammals. The other type of nucleic acid, RNA, is mostly involved in protein synthesis. DNA and RNA are made up of smaller building blocks known as nucleotides. The nucleotides combine with each other to form the larger DNA or RNA molecules. Nucleic acids also include Adenosine triphosphate (ATP), a molecule that is the source of ready energy in all cells.
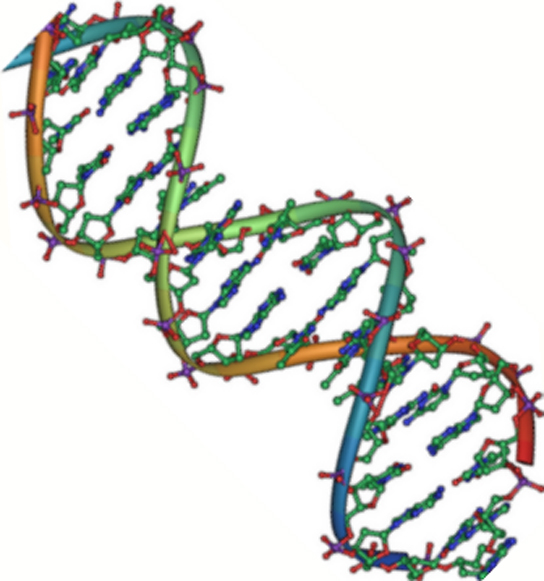
Figure 16. The double-helix model shows DNA as two parallel strands of intertwining molecules. (Credit: Jerome Walker, Dennis Myts)
Nucleic acids are described in more detail, with helpful explanations in the following video:
Bozeman Science (2012, Nov 12) Nucleic Acids [Video – YouTube] https://youtu.be/NNASRkIU5Fw
2.6 Cell Theory and Types of Cells
Cells are the building blocks of every living organism, including bacteria, yeast, mushrooms, slime molds, plants, and animals. Recall that in the organization of matter, cells are composed of organelles, organelles are composed of molecules, and molecules are composed of atoms. So cells are made up of atoms like carbon, oxygen, and nitrogen, bonded together to form molecules, like water, carbohydrates, and proteins.
The Cell Theory states the following:
all living things are composed of one or more cells
the cell is the basic unit of life
all new cells arise from existing cells
There are many types of cells, and all are grouped into one of two broad categories: prokaryotic and eukaryotic. Animal, plant, fungal, and protist cells are classified as eukaryotic, whereas bacteria and archaea cells are classified as prokaryotic.
Prokaryotic cells and eukaryotic cells differ in a lot of ways, however, all cells, including prokaryotes and eukaryotes, share four common components:
A plasma (cell) membrane – an outer covering that separates the cell’s interior from its surrounding environment
Cytoplasm – a jelly-like fluid region within the cell in which other cellular components are found
DNA – the genetic material of the cell
Ribosomes – particles that synthesize proteins
Prokaryotic Cells
Prokaryotic cells (Figure 17) are only found in simple, single-celled (unicellular) organisms, such as bacteria and archaea. Prokaryotic cells lack a nucleus and membrane-bound organelles. Prokaryotic DNA is found in a nucleoid region of the cytoplasm.
Bacteria have a cell wall made of peptidoglycan (molecules comprised of sugars and amino acids) and many have a polysaccharide capsule. The cell wall acts as an extra layer of protection, helps the cell maintain its shape, and prevents dehydration. The capsule enables the cell to attach to surfaces in its environment.
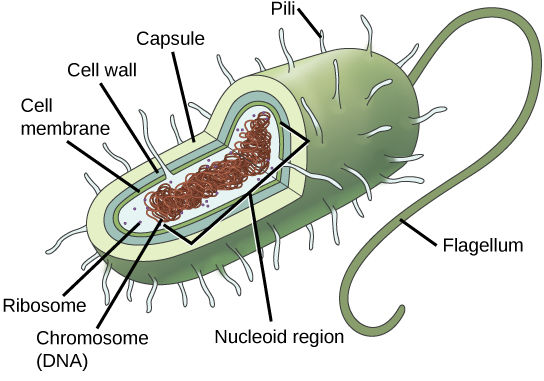
Figure 17. This figure shows the generalized structure of a prokaryotic cell. (Credit: “Prokaryotic Cell” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
Eukaryotic Cells
A eukaryotic cell (figure 1.21) is a cell that has a nucleus surrounded by a membrane and other membrane-bound structures called organelles. The word “organelle” means “little organ”. There are many different types of organelles, each with a specialized cellular function, just as the organs of your body have specialized functions. Examples of membrane-bound organelles in eukaryotic cells include mitochondria, which conduct chemical reactions to extract ATP energy from nutrients to power other cellular processes, the endoplasmic reticulum, which synthesizes lipids, carbohydrates, and modifies proteins produced by ribosomes, and the golgi apparatus, which further modifies proteins it receives from the endoplasmic reticulum, packages the proteins for export, and send them to their final destination. Eukaryotic cells are the building blocks of multicellular organisms, such as plants, animals, and fungi, and also make up some unicellular organisms, such as protists.
Animal Cells versus Plant Cells
Animals and plants are both made out of eukaryotic cells. Despite their fundamental similarities, there are some striking differences between animal and plant cells (Figure 18). Animal cells (Figure 18a) have centrioles, centrosomes, and lysosomes, whereas plant cells do not. Plant cells (Figure 18b) have a rigid cell wall that is external to the plasma membrane, chloroplasts, plasmodesmata, plastids used for storage, and a large central vacuole, whereas animal cells do not.
From an ecological perspective, chloroplasts are a particularly important type of organelle because they perform photosynthesis. Photosynthesis forms the foundation of the food chain in most ecosystems. Chloroplasts are only found in eukaryotic cells such as plants and algae. During photosynthesis, carbon dioxide (CO2), water (H2O), and light energy are used to make glucose (C6H12O6) and oxygen (O2). Chloroplasts have special structures that allow them to perform photosynthesis. They contain pigment molecules, known as chlorophyll, that are able to absorb light energy from the Sun and specialized proteins and enzymes needed for the chemical reactions to occur.
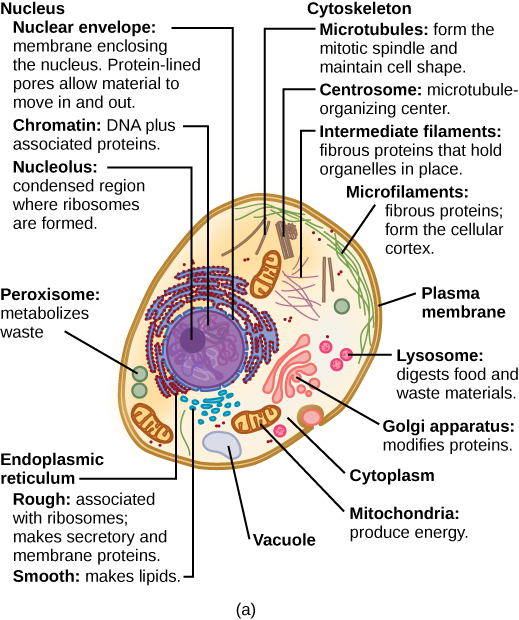
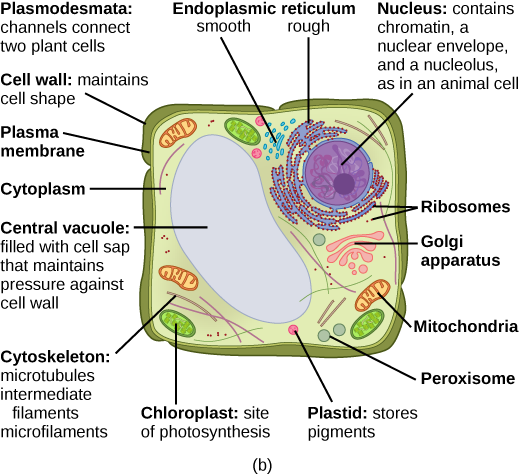
Figure 18. This figure shows (a) a typical animal cell and (b) a typical plant cell. (Credit: “Animal vs. Plant Eukaryotic Cell” by OpenStax Concepts of Biology is licensed under CC BY 4.0)
Cell Size
At 0.1–5.0 µm in diameter, most prokaryotic cells are significantly smaller than eukaryotic cells, which have diameters ranging from 10–100 µm (Figure 19). The small size of prokaryotes allows ions and organic molecules that enter them to quickly spread to other parts of the cell. Similarly, any wastes produced within a prokaryotic cell can quickly move out. However, larger eukaryotic cells have evolved different structural adaptations to enhance cellular transport. Indeed, the large size of these cells would not be possible without these adaptations. Even with these adaptations, cell size is limited because volume increases much more quickly than surface area in three-dimensional objects. As a cell becomes larger, it becomes more and more difficult for the cell to acquire sufficient materials to support the processes inside the cell, and to remove wastes, because the relative size of the surface area through which materials must be transported declines.
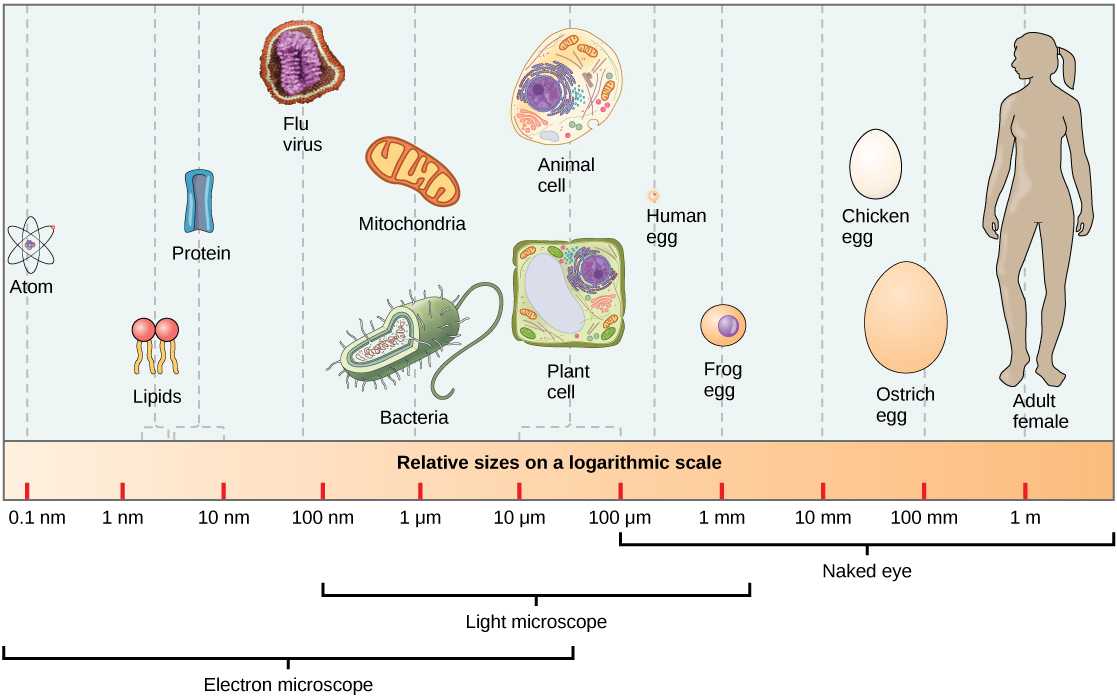
Figure 19. This figure shows the relative sizes of different kinds of cells and cellular components. An adult human is shown for comparison.
“Cell Size Comparison” by OpenStax Concepts of Biology is licensed under CC BY 4.0
For more discussion of cell size, cell organelles, and cell function, view the following video from Bozeman Science:
Bozeman Science (2012, Feb 24 ) A tour of the cell [Video – YouTube] https://youtu.be/1Z9pqST72is
Attribution:
Content in this chapter includes original work created by Lauren Roberts and Paul Bosch as well as from the following sources, with some modifications:
“Biology, 2nd edition” by OpenStax is licensed under CC BY 4.0 / A derivative from the original work

