3.2 How to Identify Minerals
Charlene Estrada
Identifying Minerals
Given the narrow criteria, you might think that Earth’s minerals are pretty rare. However, our planet (and the greater Universe) has found thousands of unique ways to combine elements into crystalline solids. There are over 5,600 minerals that have been discovered on Earth so far! We also think there are at least another thousand or more that have yet to be discovered by mineral scientists, called mineralogists.
If there are so many minerals on Earth, how does a scientist tell one apart from another? If you found the crystal cluster pictured below in a cave, how would you know whether it was gypsum and not quartz?

Physical Properties of Minerals
Minerals are categorized based on their chemical composition (see table below). Some minerals within the same compositional group will have similar physical characteristics such as color or shape. These properties are usually related to the type of elements within a mineral or how the atoms are bonded.
| Group Name | Chemical Composition | Examples |
| Native Elements | Single Elements | Sulfur, Silver, Gold |
| Silicates | SiO2 Family | Quartz, Feldspars, Micas |
| Carbonates | CO3 Family | Calcite |
| Sulfates | SO4 Family | Gypsum |
| Oxides | Oxygen Anion | Hematite, Magnetite |
| Sulfides | Sulfur Anion | Pyrite, Galena |
| Halides | Chloride or Fluoride Anion | Halite, Fluorite |
Mineralogists have identified some simple physical tests (described in the above video) to determine the identity of different minerals. These tests are ideal because prior to their use, scientists had to rely on complex (and expensive!) instruments such as X-ray Diffractometers. With these physical identification tests, most minerals can be identified in the field by scientists and nonscientists alike! The physical properties of minerals include:
Below, we will explore each of these tests in more detail. You will find that some tests will be more useful than others. Which tests will you find to be the most reliable when identifying an unknown mineral?
Color
Color is one of the first things we notice about minerals, and for good reason. Some minerals are bright with reds, greens, blues, and yellows. These colors are determined by the main elements in a mineral’s chemical formula. For example, copper (Cu), will cause azurite to be blue and malachite to be green.

Sometimes, minerals have trace elements within their structure that cause them to take on unique colors. These trace elements are often metals such as chromium (Cr), manganese (Mn), or titanium (Ti), and because they are present in such limited amounts, they do not appear in the mineral’s chemical formula.
There are certain minerals that are reliably a specific color. Malachite is green. Azurite is blue. Sulfur is yellow. Knowing these patterns makes it easier to identify certain minerals. However, there are a lot of minerals that take on a wide variety of colors. Quartz is a good example – depending on the trace elements hiding within its structure, it can be clear, white, pink, gray, black, yellow, or brown!

Streak
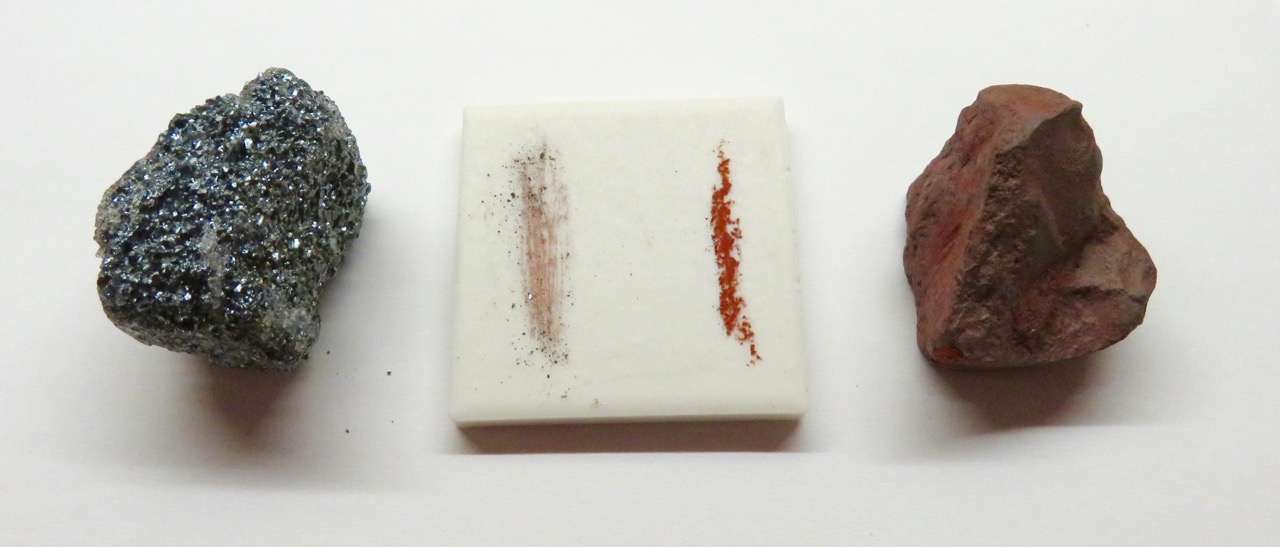
Streak is the color of a mineral’s powder. Streak is a more reliable property than color because streak does not vary between two samples of a mineral, even when those samples are different colors (see above example). Minerals that are the same color may have a different colored streak. Many minerals, such as the quartz, do not have a streak.
To check streak, scrape the mineral across an unglazed porcelain plate. Yellow-gold pyrite has a blackish streak, another indicator that pyrite is not gold, which always has a golden-yellow streak.

Luster
Luster describes the reflection of light off a mineral’s surface. Mineralogists have specific terms to describe luster. One straightforward way to classify luster is based on whether the mineral is metallic or non-metallic. Minerals that are opaque and shiny, such as pyrite, have a metallic luster.
Minerals such as quartz have a non-metallic luster, but there are still a variety of ways to describe how the light reflects off the mineral. Let’s take a look below:
| LUSTER TYPE | EXAMPLE |
| Adamantine |
 |
| Vitreous |
 |
| Silky |
 |
| Greasy |
 |
| Waxy |
 |
| Dull/ Earthy |
 |
| Metallic |
 |
Habit
A mineral’s habit is the crystal shape or texture in a given specimen. It can refer to the expression of a crystal shape or the shape of multiple crystals aggregated or bunched together. Besides color, it is often the first thing you might notice about a mineral. Crystal shapes are usually determined by the arrangement of the atoms within the crystal structure. For instance, minerals with a cubic atomic structure will have a tendency to grow into cube-shapes.
The table below displays some common mineral habits.
| HABIT TYPE | DESCRIPTION | EXAMPLE |
| Prismatic | Column-like with visible crystal faces |
 |
| Acicular | Thin, needle-like or in clusters |
 |
| Micaceous | Flat and flaky, peels apart into layers |
 |
| Botryoidal[/pb_glossary] | Bubbling or globular, circular crystals |
[caption id="attachment_1966" align="aligncenter" width="300"] |
| [pb_glossary id="1631"]Equant | Boxy or round with roughly equal dimensions |
 |
| Bladed | Elongated but flattened crystals or clusters |
 |
| Massive | Grainy, with no distinct crystals |
 |
Breakage
What does a mineral look like when it is broken? Cleavage and/or fracture describes the appearance of a mineral when a crystal is broken from an external force such as physical weathering or when you strike it with a hammer.
Cleavage is the tendency for crystals to break along planar surfaces that are parallel to atomic planes. It is common to observe:
| CLEAVAGE TYPE | EXAMPLE |
|
Basal: 1 direction of cleavage |
 |
| 2 directions of cleavage, perpendicular |
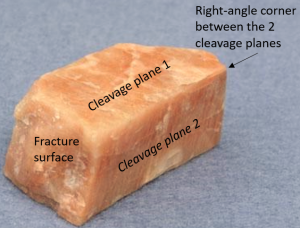 |
| 2 directions of cleavage, non-perpendicular |
 |
| Cubic: 3 directions of cleavage, perpendicular |
 |
| Rhombohedral: 3 directions of cleavage, rhombohedral |
 |
| Octahedral: 4 directions of cleavage (rare) |
 |
Some minerals do not break along smooth planes at all! Such a tendency for a mineral to break unevenly is called fracture. Metals usually fracture into jagged edges. Some minerals, such as quartz, form smooth curved surfaces when they fracture. This special type of breakage is called conchoidal fracture, and it is also seen in rocks such as obsidian and chert.

Hardness
Mineral hardness is the mineral's resistance to being scratched. The hardness of the mineral can be defined by a numerical scale, called the Mohs Hardness Scale that goes from 0-10. You can test the hardness of a mineral using everyday objects like a penny, fingernail, nail, glass, or file to find out where on the hardness scale the mineral lies.
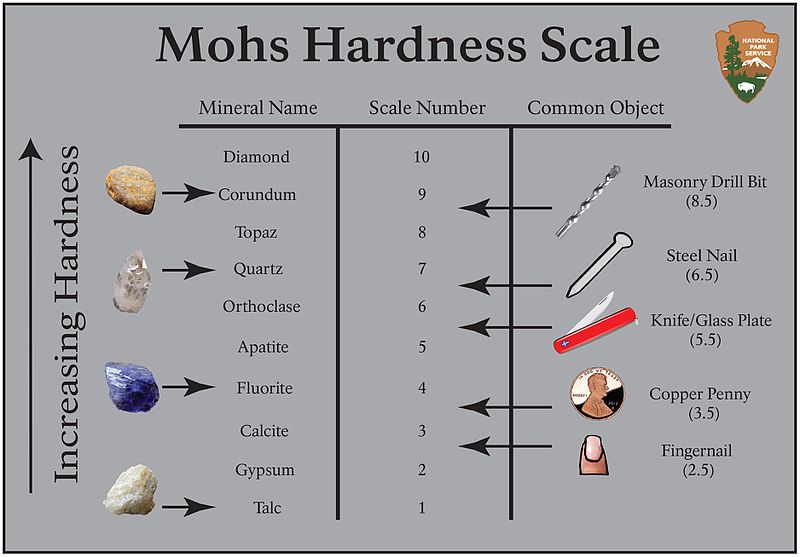
If you can scratch the mineral with the object, then the object is harder than the mineral. If the mineral can scratch the object, then the mineral is harder than the object. You can also use other minerals to assign a hardness number to a mineral. In other words, we assign a hardness number to a mineral by making relative observations!
Unique Properties
Some minerals, whether it is by chemical composition or the arrangement of their atomic structure, have unique properties that make them much more easily identifiable. Some glow brightly under UV light, others are magnetic. Some fizz when dilute acid is dripped onto their surfaces, and some are extremely dangerous! There's a wide range of unusual and interesting things that minerals can do!
Magnetism
Certain iron-bearing minerals can are strongly or weakly magnetic. The most well-known of these is the mineral for which this property is named: Magnetite.

Fluorescence
When specific minerals are exposed to high-energy ultraviolet light, the atoms within them are excited to a higher energy state. As those atoms relax back down to their default state, they'll emit light at a new wavelength, which can mean that we'll see beautiful and bright neon colors! This neat visual property is called fluorescence.

Effervescence
Effervescence is a mineral's reaction to acid. Geologists can often identify certain minerals by dripping small amounts of dilute hydrochloric acid (HCl) on them. This is called the "acid test". We often see the strongest reaction from the mineral calcite.
[Video Description: Hydrochloric acid is dripped onto the white calcite mineral and it begins to loudly fizz, foam, and bubble. Some smoke also begins to rise from the mineral as a very tiny amount of it dissolves.]
The reaction in the above video occurs because the carbonate group in calcite (CaCO3) easily dissolves into carbon dioxide (CO2) gas, which you can see by the fizzing and bubbling at the surface. Sometimes, depending on the purity of the mineral, you might even see vapor or smoke rising from the fizzing mineral!
This test is extremely useful for geologists because only carbonate materials will strongly react to the acid. Geologists can also identify some sedimentary and metamorphic rocks that contain these very same minerals based on this test.
Optical Properties
Some minerals can be completely transparent, but even if we can view images through certain crystals, they can appear different or distorted! Calcite is well-known to "double" images if you look through clear, transparent samples of this crystal. This property is called "Double Refraction".
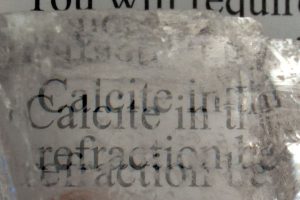
Translucent ulexite can transmit an entire image when polished. This unique ability to transmit images has given ulexite the nickname "TV Stone".

a solid, inorganic, and crystalline substance that has a predictable chemical composition and form by natural processes.
A pure substance that is not composed of any other ingredients found in the Periodic Table of Elements. One atom can be composed of only one element and form bonds with other elements.
A substance with its atoms arranged in a solid, repetitive structure.
A geoscientist that studies and characterizes minerals.
A common evaporite mineral that precipitates in dry or arid environments near evaporating water. It has the formula CaSO4 * 2H2O.
A very common rock-forming silicate mineral with formula SiO2.
The smallest unit of matter that can still form a chemical element.
A group of minerals composed only of a single chemical element.
A bright, yellow-colored mineral that often smells of rotten eggs. It is composed only of the element sulfur (S), and it often mined for its use in explosive materials.
a grayish-white metallic mineral composed only of the element silver (Ag). It is a precious metal used by the global economy.
a metallic, brassy-yellow mineral made only of the element gold (Au). It is a precious metal in the world economy.
A group of minerals composed of silica in the form of Si and O in some combination. The most abundant mineral group on and within the Earth.
A group of silicate minerals which represents the most abundant mineral class of the continental crust.
A group of silicate minerals called the "sheet" silicates. Mica minerals have their atomic structure arranged to easily pull apart as sheets in one direction.
A mineral group composed of the carbonate (CO3--) anion.
CaCO3
A carbonate mineral that strongly reacts with dilute acid. Calcite composes the sedimentary rock limestone and composes the skeletons of some ocean life.
A mineral group composed of the sulfate (SO4--) anion.
A class of minerals with a chemical composition of a metallic element bound with oxygen.
A negatively charged atom.
Fe2O3
A gray to rust-brown oxide mineral that is iron (Fe)-rich. The reddish color forms from the oxidation, or rusting, of iron by the atmosphere.
Fe2+Fe3+2O4
A dark gray, oxide mineral that is iron-rich. Magnetite is strongly magnetic and attracts iron-rich materials.
A mineral compound characterized by the linkage of sulfur with a metal or semi-metal such as galena, PbS, or pyrite, FeS2.
FeS2
A brassy yellow metallic mineral from the sulfide class. Pyrite is also known as "Fool's Gold" for its golden color.
PbS
A gray, metallic mineral of the sulfide class. Galena is very dense and feels heavy when held.
A mineral group composed of the chloride (Cl-) or fluoride (F-) anion.
NaCl
A salty, halide mineral that is often colorless or white. Halite is commonly known as rock salt and used globally as a condiment. It tends to form as cubic-shaped grains or crystals.
CaF2
A halide mineral that often fluoresces under ultraviolet light. Fluorite can be almost any color, and it will sometimes form octahedrally-shaped crystals.
An instrument that can determine the spacing of atoms in a crystal structure using X-ray radiation in order to identify a particular mineral.
The color of a mineral when crushed or scratched into a powder.
The way in which light reflects from a mineral's surface. The observation of luster can help identify a mineral.
The crystal shape or texture of a mineral. Some minerals tend to have distinctive habit and can be identified using this criteria.
a measure of a mineral's durability or resistance to being scratched.
Cu3(CO3)2(OH)2
A blue, copper carbonate mineral that is sometimes used for decorative purposes.
Cu2CO3(OH)2
A green, copper-carbonate mineral that is sometimes used for decorative purposes.
A luster that has the appearance of a polished metal surface reflecting light.
Brilliant, very reflective version of glassy luster. Often applies to diamonds and other highly polished gemstones.
Reflective and glassy luster.
A nonmetallic luster with a cloth-like sheen or is finely-fibrous.
A nonmetallic luster that appears oily.
A nonmetallic luster with a candle-like appearance that weakly reflect light on its surface.
A nonmetallic luster that barely or does not appear to have any shine to its surface. Also called dull.
A solid in which the atoms are arranged into a regular, repeating pattern.
Habit displaying long or column-like crystal shape with distinctive faces.
Habit in which crystals grow in long, thin needles or cluster together in long, thin needles.
Habit in which minerals grow in flat, flaky layers that easily peel apart. Common to the mica mineral group.
Habit in which crystals appear to be bubbling or as clusters of globes or semicircles.
Habit in which crystals are elongated and flattened and can grow alone or in clusters.
Habit in which there are no distinctive crystal shapes; the substance is fine and grainy. Also known as granular.
the breakage of minerals along smooth, planar surfaces that are parallel to atomic planes in its structure.
the breakage of a mineral (or sometimes, a rock) that is not along a cleavage plane.
A type of weathering that involves the abrasion and breakdown of existing rocks and minerals by water, the atmosphere, and biosphere. Also known as mechanical weathering.
A type of cleavage in which the mineral splits along a single plane or direction.
A type of 3-directional cleavage found in minerals in which each cleavage plane is at 90 degrees from one another.
Three directional cleavage found in minerals, in which the cleavage directions are not perpendicular to one another.
Four-directional cleavage found on minerals.
The breakage of a rock or mineral that forms smooth, curved surfaces.
A felsic, extremely smooth, fine-grained igneous rock that is glassy in texture. Obsidian is primarily composed of amorphous silica and displays conchoidal fracture.
A chemical, or sometimes biological, sedimentary rock made of microcrystalline silica that has precipitated from water.
The numerical scale, from 0-10, that measures the hardness of a mineral. "0" is a very soft, crumbly mineral and "10" is diamond-hard.
A high energy light that is bluish-violet to non-visible. UV light can damage biological structures but also propel atoms to a higher energy state to cause fluorescence. UV lights are sometimes called "blacklights".
The property of a substance (e.g. a rock or mineral) to emit light appearing as different colors when it is exposed to a much higher intensity light, such as ultraviolet radiation.
A substance's (e.g. rock or mineral) tendency to fizz and bubble, which indicates the release of gas.
rocks that cement together from weathering products, either from sediments or chemical ions in water.
Rocks that form when any type of preexisting rock is warped or transformed under elevated temperatures and pressures.
A property in which a crystal bends incoming light into two rays that causes images to appear doubled.
A material that allows the transmission of light, but details of objects on the other side are not clearly visible.


