GENETIC ENGINEERING
“Genetic engineering is the most powerful and awesome skill acquired by man since the splitting of the atom”.
Time magazine
restriction enzymes
Genetic engineering is defined as alteration of the genetic material in an organism to change its traits or to allow the organism to produce a biological product. Genetic engineering began in the 1970s when we were able to manipulate DNA. One of the products of genetic engineering is recombinant DNA; DNA spliced together from two or more organisms. We have spliced together human and Escherichia coli DNA. Restriction enzymes are critical in producing recombinant DNA. Restriction enzymes were discovered in bacteria living in old faithful. More than 3000 restriction enzymes have been studied and more than 600 are available commercially. A restriction enzyme cuts DNA after a specific nucleotide sequence. Because the order of nitrogenous bases is unique each person’s DNA will be cut into a different number of fragments.
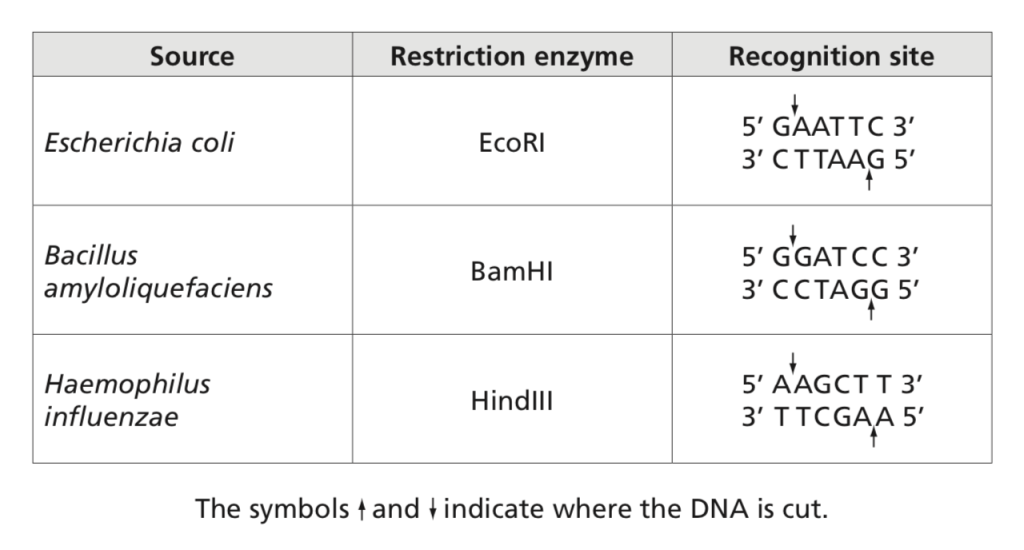
Restriction enzymes can be used to cut a human gene out human DNA. We can isolate a plasmid from E. coli and open the plasmid with the same restriction enzyme. We then insert the human gene into the plasmid and knit the DNA together using DNA ligase. The plasmid containing the human gene is inserted into E. coli via transformation; E. coli will transcribe and translate the human gene. Recombinant DNA technology is used to produce human proteins including insulin, clotting factors, interferon, and growth hormone. Recombinant bovine growth hormone increases milk production by 41%. Recombinant DNA technology is also used to manipulate organisms to produce larger amounts of antibiotics, add herbicide or pesticide resistance to plants, and produce vaccines (hepatitis B and Gardasil).
Mechanism of recombination animation hyperlink
DNA fingerprints
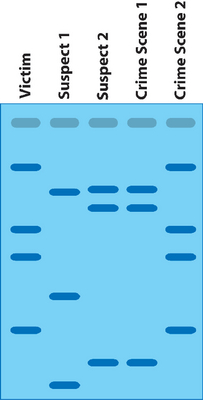
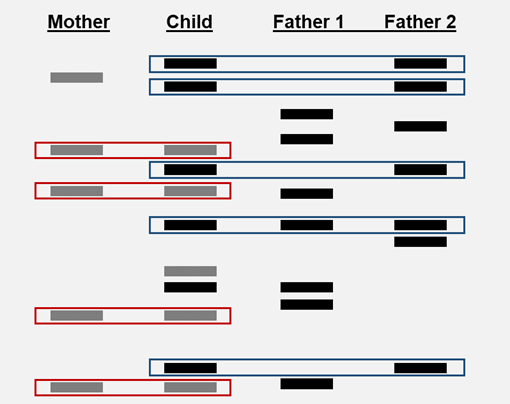
Restriction enzymes are also used to produce DNA fingerprints. DNA fingerprints can be used in crime scene analysis and paternity testing. The DNA samples are digested with a restriction enzyme. Because the order of nitrogenous bases is unique each person’s DNA will be cut into a different number of fragments. This is known as RFLP or restriction fragment length polymorphism. The process of gel electrophoresis separates the DNA fragments according to size and creates a pattern. In crime scene analysis the patterns are compared; the suspect pattern must match the crime scene sample pattern. In paternity testing the child inherits half its DNA from mom and half from dad. When a paternity gel is interpreted you eliminate the DNA that mom gave the child; dad has to give the child the rest of the DNA.
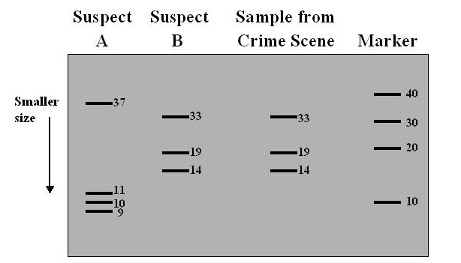
The banding pattern of suspect B matches the banding pattern of the sample from the crime scene. Suspect B is guilty.
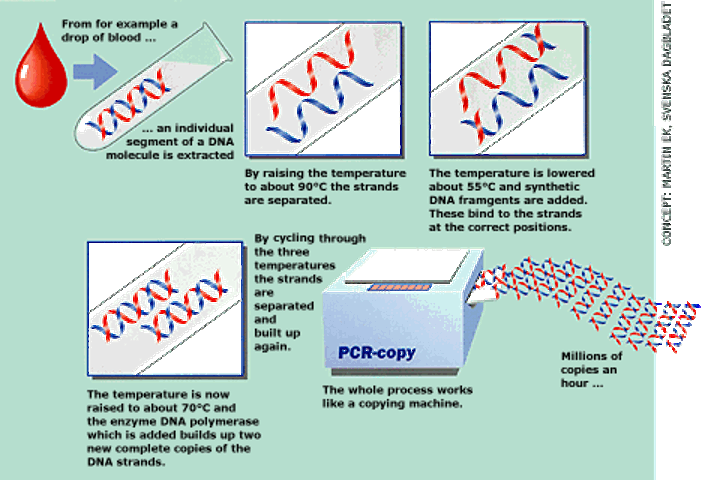
polymerase chain reaction (pcr)
Polymerase chain reaction (PCR) is used to multiply DNA. You can start with a single copy of DNA and generate billions of exact copies within hours. PCR can be used in crime scene analysis if the crime scene sample is too small for further testing. PCR is also used for diagnostic testing (HIV, West Nile virus, Lyme disease).
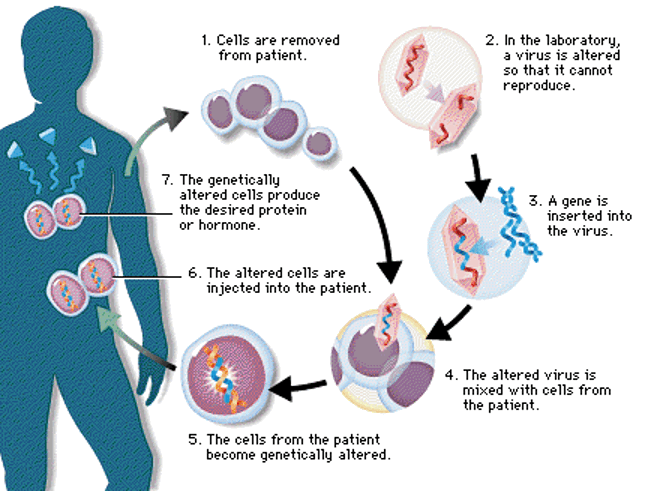
gene therapy
Gene therapy is a technique for correcting defective genes responsible for disease development. In most gene therapy studies, a normal gene is inserted into the genome to replace an abnormal, disease-causing gene. A carrier molecule called a vector must be used to deliver the therapeutic gene to the patient’s target cells. Currently, the most common vector is a virus that has been genetically altered to carry normal human DNA. The virus unloads its genetic material containing the therapeutic human gene into the target cell. The generation of a functional protein product from the therapeutic gene restores the target cell to a normal state.
Besides virus-mediated gene-delivery systems, there are several non-viral options for gene delivery. The simplest method is the direct introduction of therapeutic DNA into target cells. This approach is limited in its application because it can be used only with certain tissues and requires large amounts of DNA. Another non-viral approach involves the creation of an artificial lipid sphere with an aqueous core. This liposome, which carries the therapeutic DNA, is capable of passing the DNA through the target cell’s membrane. Researchers also are experimenting with introducing a 47th (artificial human) chromosome into target cells. This chromosome would exist autonomously alongside the standard 46 not affecting their workings or causing any mutations. It would be a large vector capable of carrying substantial amounts of genetic code, and scientists anticipate that, because of its construction and autonomy, the body’s immune systems would not attack it. A problem with this potential method is the difficulty in delivering such a large molecule to the nucleus of a target cell.
What factors have kept gene therapy from becoming an effective treatment for genetic disease? Before gene therapy can become a permanent cure for any condition, the therapeutic DNA introduced into target cells must remain functional and the cells containing the therapeutic DNA must be long-lived and stable. Problems with integrating therapeutic DNA into the genome and the rapidly dividing nature of many cells prevent gene therapy from achieving any long-term benefits. Patients will have to undergo multiple rounds of gene therapy. Anytime a foreign object is introduced into human tissues, the immune system is designed to attack the invader. The risk of stimulating the immune system in a way that reduces gene therapy effectiveness is always a potential risk. Furthermore, the immune system’s enhanced response to invaders it has seen before makes it difficult for gene therapy to be repeated in patients. Viruses, while the carrier of choice in most gene therapy studies, present a variety of potential problems to the patient (toxicity, immune and inflammatory responses, and gene control and targeting issues). In addition, there is always the fear that the viral vector, once inside the patient, may recover its ability to cause disease. Conditions or disorders that arise from mutations in a single gene are the best candidates for gene therapy. Unfortunately, some the most commonly occurring disorders, such as heart disease, high blood pressure, Alzheimer’s disease, arthritis, and diabetes, are caused by the combined effects of variations in many genes. Multi-gene or multi-factorial disorders such as these would be especially difficult to treat effectively using gene therapy. Here are a few gene therapy videos:
human genome project
The Human Genome Project (HGP) was the international, collaborative research program whose goal was the complete mapping and understanding of all the genes of human beings. All our genes together are known as our “genome.” HGP researchers have deciphered the human genome in three major ways: determining the order, or “sequence,” of all the bases in our genome’s DNA; making maps that show the locations of genes for major sections of all our chromosomes; and producing what are called linkage maps through which inherited traits (such as those for genetic disease) can be tracked over generations.
The HGP has revealed that there are probably about 20,500 human genes. The completed human sequence can now identify their locations. This ultimate product of the HGP has given the world a resource of detailed information about the structure, organization and function of the complete set of human genes. This information can be thought of as the basic set of inheritable “instructions” for the development and function of a human being. The International Human Genome Sequencing Consortium published the first draft of the human genome in the journal Nature in February 2001 with the sequence of the entire genome’s three billion base pairs some 90 percent complete. A startling finding of this first draft was that the number of human genes appeared to be significantly fewer than previous estimates, which ranged from 50,000 genes to as many as 140,000. The full sequence was completed and published in April 2003.
Upon publication of the majority of the genome in February 2001, Francis Collins, the director of NHGRI, noted that the genome could be thought of in terms of a book with multiple uses: “It’s a history book – a narrative of the journey of our species through time. It’s a shop manual, with an incredibly detailed blueprint for building every human cell. And it’s a transformative textbook of medicine, with insights that will give health care providers immense new powers to treat, prevent and cure disease.”
QuIZ TIME!
