Silicate Minerals
Karla Panchuk
Silicon and oxygen bond covalently to create a silicate tetrahedron (SiO44-), which is a four-sided pyramid shape with oxygen at each corner and silicon in the middle (Figure below). This structure is the building block of many important minerals in the crust and mantle. Silicon has a charge of +4, and oxygen has a charge of -2, so the total charge of the silicate anion is -4.
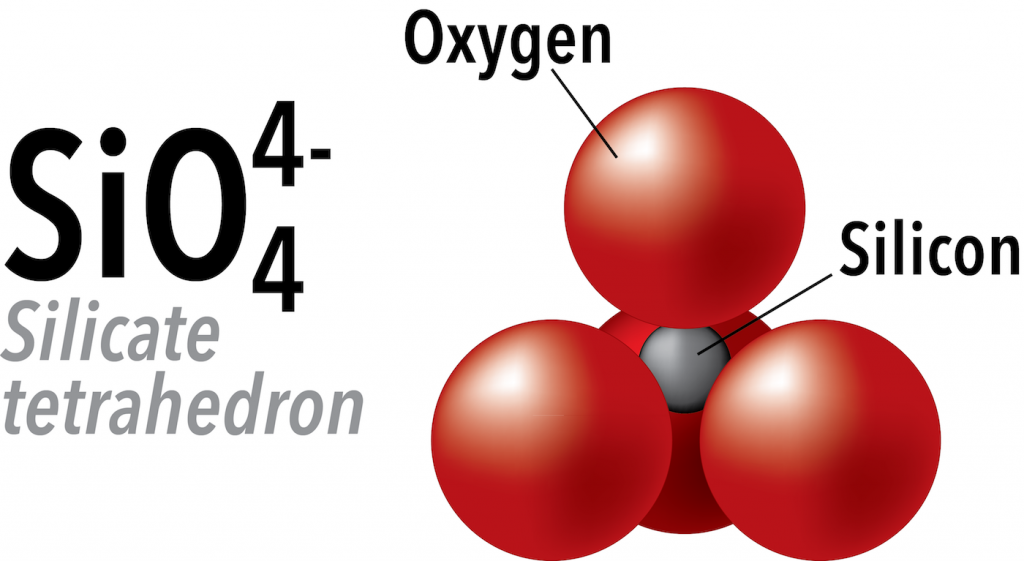
In silicate minerals, these tetrahedra are arranged and linked together in various ways, from single units to chains, rings, and more complex frameworks. In the rest of this section, we will look at the structures of the most common silicate minerals in Earth’s crust and mantle.
Isolated Tetrahedra
The simplest silicate structure—that of the mineral olivine (Figure below)—is composed of isolated tetrahedra bonded to iron and/or magnesium ions. In olivine, the –4 charge of each silica tetrahedron is balanced by two iron or magnesium cations, each with a charge of +2.

Olivine can be pure Mg2SiO4 or pure Fe2SiO4, or a combination of the two, written as (Mg,Fe)2SiO4.
Chain Silicates
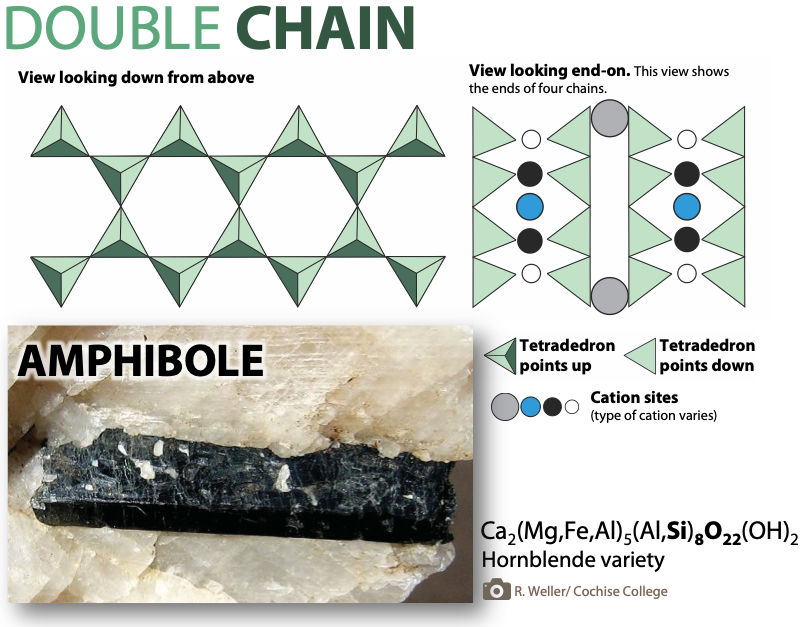
The mineral pyroxene is an example of a single-chain silicate (Figure 5.25), where one oxygen from each tetrahedron is shared with the next tetrahedron. Sharing means that fewer oxygens are needed to make the tetrahedra, so there’s less oxygen in this structure compared to olivine. This can be expressed as a silicon-to-oxygen ratio (Si:O).
In olivine, tetrahedra aren’t connected directly to each other, so each silicon atom must have four oxygen atoms all to itself to complete a tetrahedron. That’s a ratio of 1:4. In pyroxene, each silicon atom only needs three unique oxygen atoms because it can borrow one from a neighbor to have a tetrahedron. For pyroxene, the Si:O is 1:3. With one less oxygen in the mix per tetrahedron, the net charge per silicon atom that cations must balance is lower (-2 instead of -4).

The way tetrahedra share oxygens in single-chain silicates is why, even though pyroxene is built out of silicate tetrahedra (with the silicate anion being SiO44-), its formula has the tetrahedra represented as SiO3 (e.g., MgSiO3, FeSiO3, and CaSiO3.
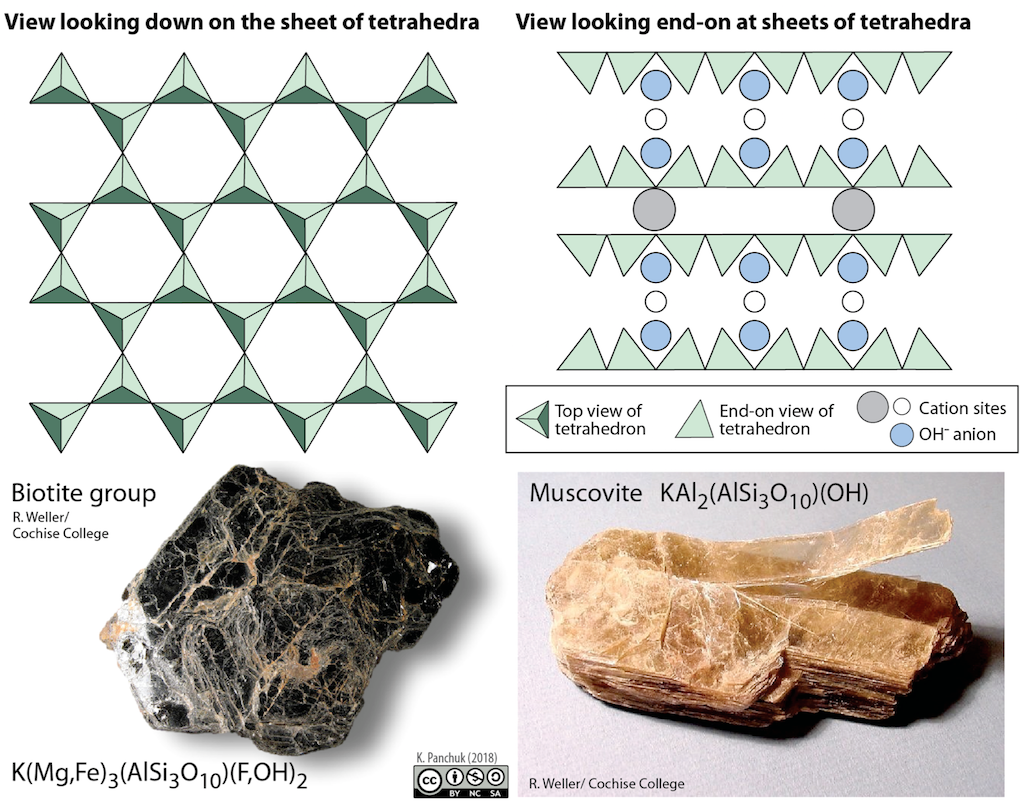
In mica structures, the silica tetrahedra are arranged in continuous sheets (Figure below), where each tetrahedron shares three oxygen anions with adjacent tetrahedra. Because even more oxygens are shared between adjacent tetrahedra, fewer charge-balancing cations are needed for sheet silicate minerals.
Bonding between sheets is relatively weak, which accounts for the tendency of mica minerals to split apart in sheets (Figure below, to the bottom right). Two common micas in silicate rocks are biotite (Figure below, to the bottom left), which contains iron and/or magnesium, making it a dark mineral, and muscovite (Figure below on the right), which contains aluminum and potassium and is light in color. All of the sheet silicate minerals have water in their structure, in the form of the hydroxyl (OH-) anion.
Some sheet silicates typically occur in clay-sized fragments (i.e., less than 0.004 mm). These include the clay minerals kaolinite, illite, and smectite, which are important components of rocks and especially of soils.
Framework Silicates
In framework silicates, tetrahedra are connected in three-dimensional structures rather than in two-dimensional chains and sheets.
Feldspar
Feldspars are a group of very abundant framework silicates in Earth’s crust. They include alumina tetrahedra as well as silicate tetrahedra. In alumina tetrahedra, an aluminum cation is at the center instead of a silicon cation.
Feldspars are classified using a ternary (3-fold) system with three end-members (“pure” feldspars). This system is illustrated with a triangular diagram with each end member at one corner (Figure below). The distance along a side of the diagram represents the relative abundance of the composition of each end member.

One end-member is potassium feldspar (also referred to as K-feldspar), which has the composition KAlSi3O8. Another end member is albite, which has sodium instead of potassium (formula NaAlSi3O8). As is the case for iron and magnesium in olivine, there is a continuous range of compositions between albite and orthoclase. Feldspars in this series are referred to as alkali feldspars.
The third end-member is anorthite, which has calcium instead of potassium or sodium (formula CaAl2Si3O8). Feldspars with a mixture of sodium and calcium in their composition are called plagioclase feldspars.
Quartz
Quartz (SiO2; Figure below) contains only silica tetrahedra. In quartz, each silica tetrahedron is bonded to four other tetrahedra (with oxygen shared at every corner of each tetrahedron), making a three-dimensional framework. As a result, the ratio of silicon to oxygen is 1:2. The hardness of quartz and the fact that it breaks irregularly (notice the bottom of the crystal in the right of the figure below) and not along smooth planes result from the strong covalent/ionic bonds characteristic of the silica tetrahedron.

References
Klein, C. & Hurlbut, C. S., Jr. (1993). Manual of Mineralogy (after J. D. Dana). New York, NY: John Wiley & Sons, Inc.

