Bonding and Lattices
Karla Panchuk
Atoms seek to have a full outer shell. For hydrogen and helium, a full outer shell means two electrons. For other elements, it means 8 electrons. The outer shell is filled by transferring or sharing electrons with other atoms in chemical bonds. The type of chemical bond is important for the study of minerals because the type of bond will determine many of a mineral’s physical and chemical properties.
Ionic Bonds
Consider the example of halite again, which is made up of sodium (Na) and chlorine (Cl). Na has 11 electrons: two in the first shell, eight in the second, and one in the third (see the top half of the figure below). Na readily gives up the third shell electron so it can have the second shell with 8 electrons as its outermost shell. When it loses the electron, the electron’s total charge is -10, but the proton’s total charge is +11, so it is left with a +1 charge overall.

Chlorine has 17 electrons: two in the first shell, eight in the second, and seven in the third. Cl readily accepts an eighth electron to fill its third shell, and therefore becomes negatively charged because it has a total charge of -18 from electrons, and a total charge of +17 from protons.
In changing their number of electrons, these atoms become ions—the sodium loses an electron to become a positive ion or cation,[1] and the chlorine gains an electron to become a negative ion or anion (look at the bottom half of the figure above). Because negative and positive charges attract, sodium and chlorine ions stick together, creating an ionic bond. In an ionic bond, electrons can be thought of as having transferred from one atom to another.
Cation or Anion?
Covalent Bonds
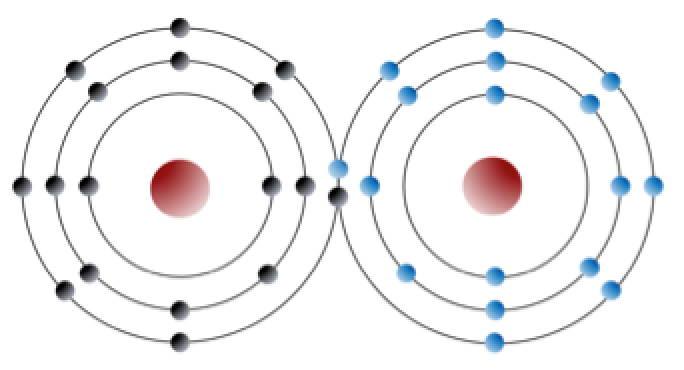
Some elements can also form bonds by sharing electrons rather than giving away or receiving them. This will happen when electrons are held especially tightly by their atoms. Chlorine gas (Cl2, Figure below) is formed when chlorine atoms share two outer-shell electrons so that each has a complete outer shell. This is called a covalent bond.
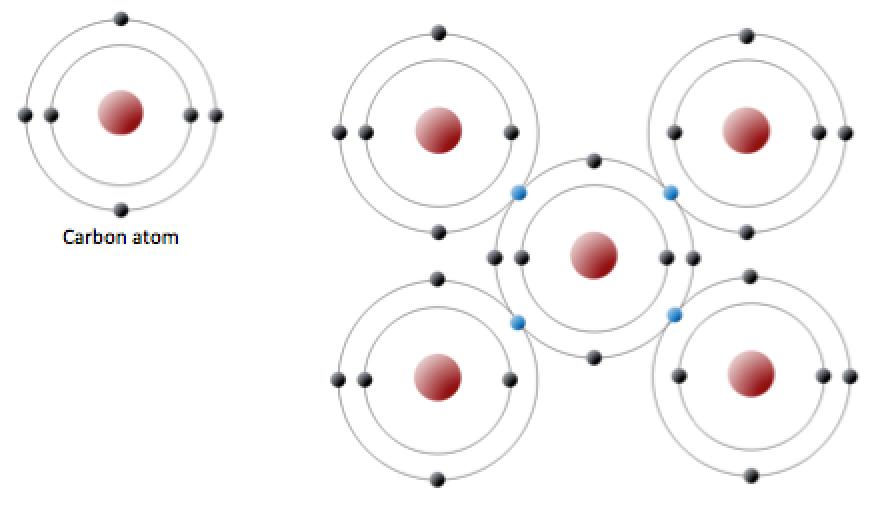
Carbon is another atom that participates in covalent bonding. An uncharged carbon atom has six protons and six electrons. Two of the electrons are in the inner shell and four are in the outer shell (left half of figure below). Carbon would need to gain or lose four electrons to have a filled outer shell, creating too great a charge imbalance. Instead, carbon atoms share electrons to create covalent bonds (see right half of the figure below).
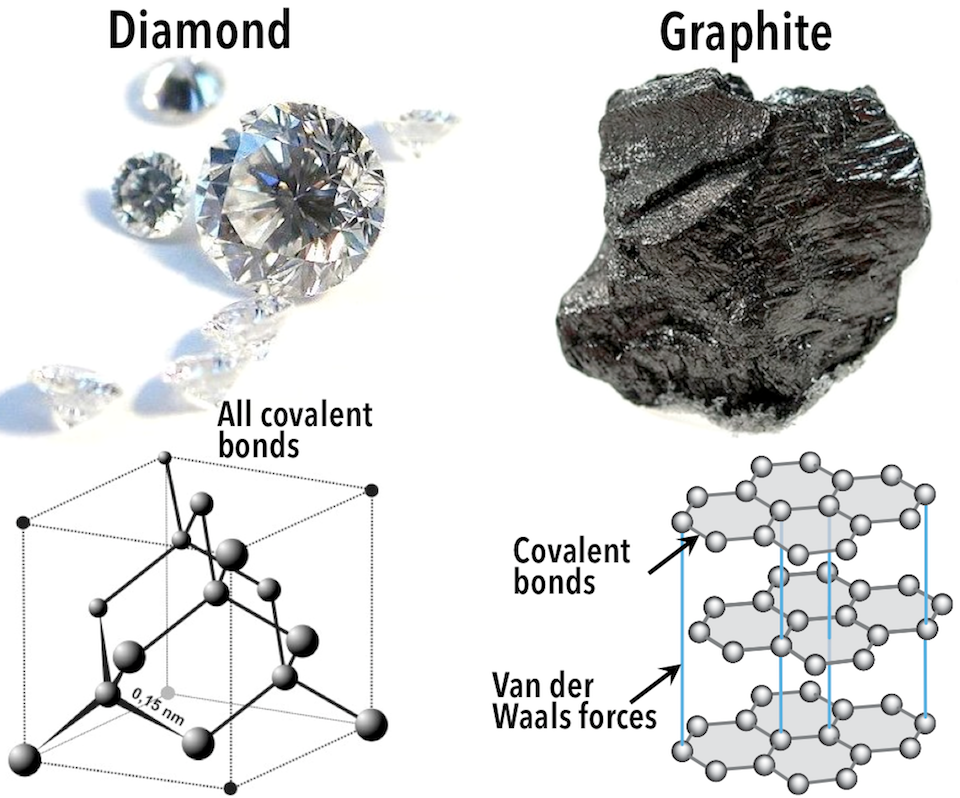
Carbon atoms are linked in a three-dimensional framework in the mineral diamond (shown on the left-hand side of the figure below). Each carbon atom is bonded to four other carbon atoms. Every bond is a very strong covalent bond.
Other Types of Bonds
Most minerals are characterized by ionic bonds, covalent bonds, or a combination of the two, but other types of bonds are important in minerals.
Van der Waals Forces and Hydrogen Bonds
Consider the mineral graphite (Figure above, right-hand side): carbon atoms are linked in sheets or layers in which each carbon atom is covalently bonded to three others. Graphite-based compounds are strong because of the covalent bonding between carbon atoms within each layer, which is why they’re used in high-end sports equipment such as ultralight racing bicycles. However, Graphite is soft because the layers themselves are held together by relatively weak Van der Waals forces.
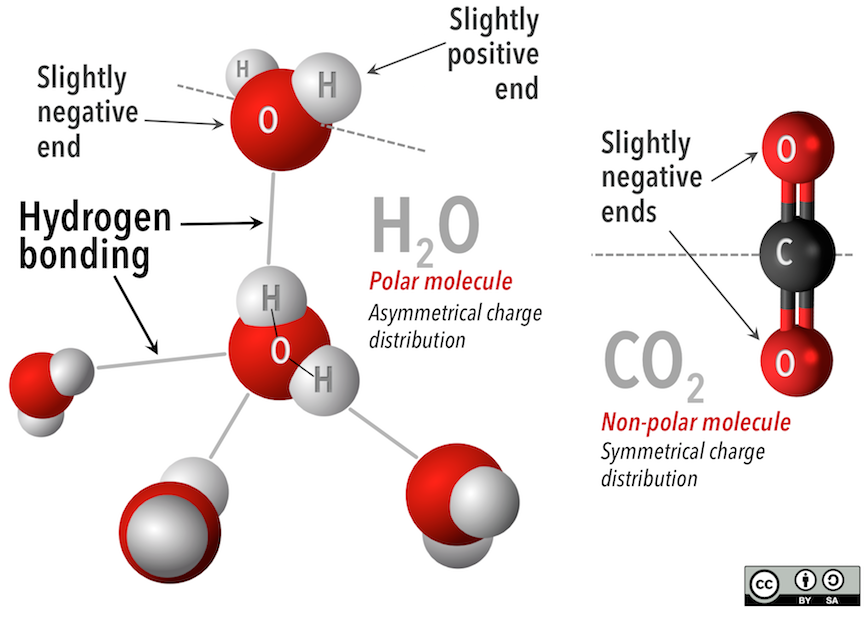
Van der Waals forces, like hydrogen bonds, work because molecules can be electrostatically neutral but still have an end that is slightly more positive and an end that is slightly more negative. In water molecules (Figure below, left-hand side), the bent shape puts the hydrogen atoms on one side of the molecule, and the oxygen atom, with more electrons, on the other. The charge is distributed asymmetrically across the water molecule. Contrast this with the straight carbon dioxide molecule (Figure below, right-hand side). The slightly more negative oxygen atoms on the ends are distributed symmetrically on either side of the carbon atom.
Metallic Bonds
Metallic bonding occurs in metallic elements because they have outer electrons that are relatively loosely held. (The metals are highlighted on the periodic table in Appendix 1.) When bonds between such atoms are formed, the dissociated electrons can move freely from one atom to another. This feature accounts for two very important properties of metals: their electrical conductivity and their malleability (they can be deformed and shaped).

Chemical Bond Types
- You can remember that a cation is positive by remembering that a cat has paws (paws sounds like "pos" in "positive"). You could also think of the "t" in "cation" as a plus sign. ↵

