Mineral Properties
Karla Panchuk
Minerals are universal. A crystal of hematite on Mars will have the same properties as one on Earth and the same as one on a planet orbiting another star. That’s good news for geology students who are planning interplanetary travel because they can use the same properties to identify minerals anywhere. That doesn’t mean that it’s easy, however. Identification of minerals takes practice. Some of the mineral properties that are useful for identification are color, streak, luster, hardness, habit, cleavage or fracture, and density.
Color
Some minerals have distinctive colors that are useful as diagnostic criteria. The mineral sulfur (Figure below, to the left) is always a characteristic bright yellow. For other minerals, the color might vary. Hematite is an example of a mineral for which color is not necessarily diagnostic. In some forms, hematite is a deep dull red (a fairly unique color), but in others, it is a metallic silvery black (Figure below, to the right).
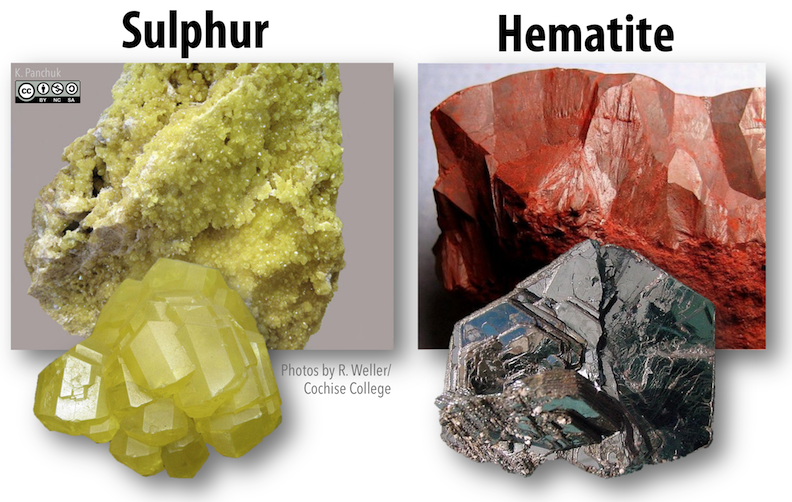
For other minerals, the problem is that a single mineral can have a wide range of colors. The color variations can result from varying proportions of trace elements within the mineral or structural defects within the crystal lattice. In the case of quartz (Figure below), milky quartz gets its white color from millions of tiny fluid-filled cavities. Smoky quartz gets its grey color from structural damage caused by natural radiation. Amethyst and citrine get their colors from trace amounts of iron, and rose quartz gets its pink hue from manganese.

Streak
The color of a mineral is what you see when light reflects off the sample’s surface. One reason that color can be so variable is that the surface texture is variable. A way to get around this problem is to grind a small amount of the sample to a powder and observe the color of the powder. This color is the mineral’s streak. The mineral can be powdered by scraping the sample across a piece of unglazed porcelain called a streak plate. In the figure below, two hematite samples have been scraped across the streak plate. Even though one sample is metallic and the other is deep red, both have a similar reddish-brown streak.

Streak is an especially helpful property when minerals look similar. The next figure below shows four different minerals, but all are silvery-black in color, with varying degrees of metallic sheen. The streaks of these minerals are much more distinctive, ranging from dark grey to yellowish brown.

Luster
Luster is how light reflects off a mineral’s surface and the degree to which it penetrates the interior. The main distinction is between metallic luster and non-metallic luster. Light doesn’t pass through metals, which is why they look metallic (e.g., look at hematite in the figure above). Even a thin sheet of metal—aluminum foil, for example—will not permit light to pass through it. Many non-metallic minerals may look as if the light will not pass through them, but if you look closely at a thin slice of the mineral, you’ll see that it is translucent or transparent.
If a non-metallic mineral has a shiny, reflective surface, it is said to have a glassy luster. The quartz crystals in the previous section are examples of minerals with glassy luster. If the mineral surface is dull and non-reflective, it has an earthy luster (like the reddish brown hematite in the previous section on Streak). Other types of non-metallic lusters are silky, pearly, and resinous (like amber or candied pineapple). Luster is a good diagnostic property because most minerals will always appear either metallic or non-metallic, although, as the two hematite samples show, there are exceptions.
Practice with Luster
Hardness
One of the most important diagnostic properties of a mineral is its hardness. In practical terms, hardness determines whether or not a particular material can scratch a mineral.
In 1812 German mineralogist Friedrich Mohs came up with a list of 10 minerals representing a wide range of hardness and numbered them 1 through 10 in order of increasing hardness (see graph below, on the horizontal axis). While each mineral on the list is harder than before, the measured hardness (vertical axis) is not linear. Notice the difference in hardness between talc and gypsum, then compare that to the difference between corundum and diamond.

Some commonly available reference materials are also shown on this diagram and summarized in the table below. Note that fingernails are often included as reference material for testing minerals, but this only applies to natural fingernails. Artificial fingernails may be much harder than natural fingernails. Some materials used for artificial nails are harder than quartz.
| Table Mohs Hardness of Scale |
||
| Mohs Hardness | Mineral | Common Reference Materials |
| 1 | Talc (softest) | |
| 2 | Gypsum | 2-2.5 – Natural fingernail |
| 3 | Calcite | 3 – Copper |
| 4 | Fluorite | 4 – Nail (steel) |
| 5 | Apatite | 5-5.5 – Glass plate, knife blade |
| 6 | Orthoclase Feldspar | 6.5-7 – Streak plates, hardened steel file |
| 7 | Quartz | |
| 8 | Topaz | |
| 9 | Corundum | |
| 10 | Diamond (hardest) | |
Using these materials to determine hardness involves testing to see which material will or won’t scratch the mineral so you can find upper and lower limits on hardness. For example, if you have a mineral that you can’t scratch with your fingernail but can scratch with a copper wire, its hardness is between 2.5 and 3. A mineral with known hardness can be used to test other minerals.
Concept Check: Testing for Hardness
Crystal Habit
When minerals form within rocks, there is a possibility that they will form in distinctive crystal shapes if other pre-existing minerals do not crowd them out. Every mineral has one or more distinctive crystal habits determined by its atomic structure, although it is not that common in ordinary rocks for the shapes to be obvious.
Quartz, for instance, will form six-sided prisms with pointed ends (see the left side of the figure below), but this typically happens only when it crystallizes from a hot water solution within a cavity in an existing rock. Pyrite can form cubic crystals (center of the figure below), but can also form crystals with 12 faces, known as dodecahedra. The mineral garnet also forms many-sided crystals with an overall rounded shape (right on the figure below).
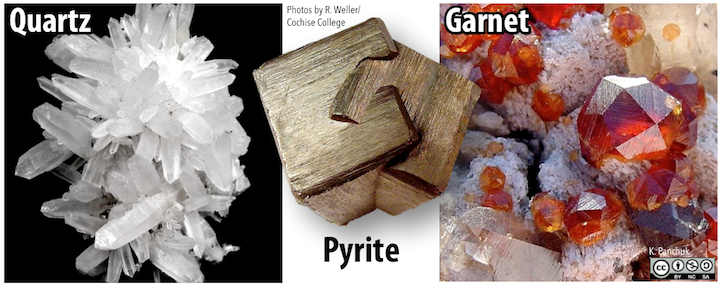
Some of the terms that are used to describe habit include bladed, botryoidal (grape-like), dendritic (branched), drusy (an encrustation of crystals), and equant (similar size in all dimensions), fibrous, platy, prismatic (long and thin), and stubby.
Cleavage and Fracture
Cleavage and fracture describe how a mineral breaks. These characteristics are the most important diagnostic features of many minerals and often the most difficult to understand and identify. We see cleavage when a mineral breaks along a plane or planes, while the fracture is an irregular break. Some minerals tend to cleave along planes at various fixed orientations. Some, like quartz, do not cleave at all, only fracture. Minerals with cleavage can also fracture along surfaces that are not parallel to their cleavage planes.
The way minerals break is determined by the atoms’ arrangement and, more specifically, by the orientation of weaknesses within their crystal lattice. Graphite and mica break off in parallel sheets (Figure below).

Other minerals have two directions of cleavage, classified as two directions at 90° and two directions not at 90°. While the diagrams of planes on the left of Figure below make this difference clear, it may be less obvious in practice. The minerals in Figure both have two planes of cleavage that are very close to 90°. The white dashed lines mark the edges of the planes, as in Figure shown above. See if you can find the planes repeated in the images. The images are close-up views of the minerals, only a few cm across. Sometimes you must look very carefully to find cleavage planes.

Some minerals have many directions of cleavage. Figure 5.38 shows minerals with three directions of cleavage. Halite (shown at the top of the figure below) has three directions at 90°, and calcite (as shown by the bottom half of the figure below) has three directions, not at 90°.
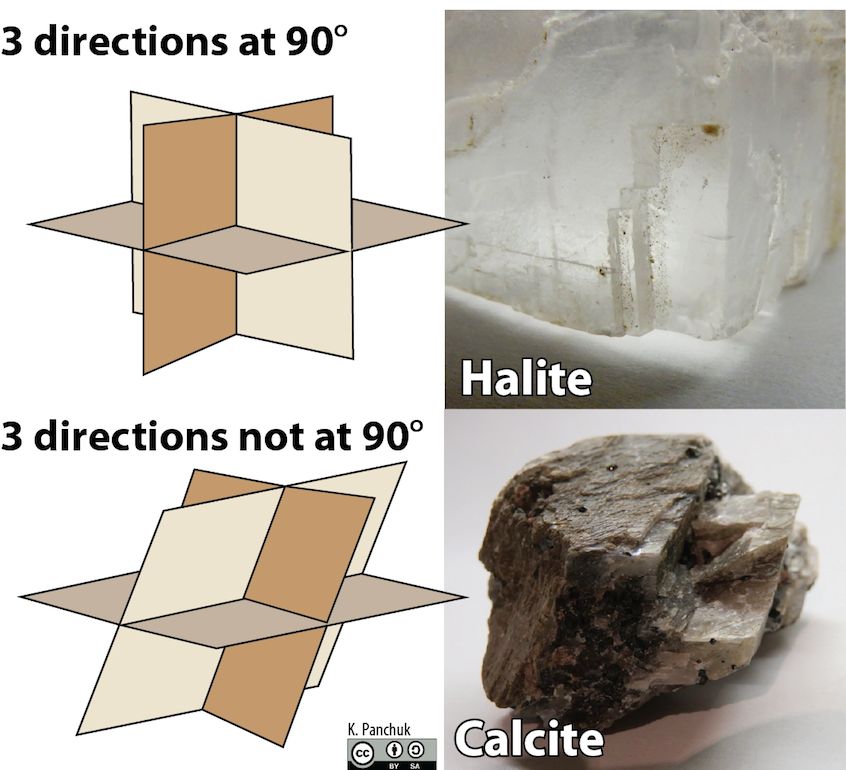
Students encounter a few common difficulties when learning to recognize and describe cleavage. One is that it might be necessary to look very closely at a sample to see mineral cleavage. The key features of cleavage are only cm or mm in scale. If crystals are very small, it may not be possible to see cleavage at all. Another issue is that sometimes cleavage is present, but it is poor, meaning the cleavage surface isn’t perfectly flat. Finally, it can be difficult to know whether a flat surface on a crystal is a cleavage plane, a crystal face, or simply a surface that happens to be flat.
Cleavage planes tend to repeat themselves at different depths throughout the mineral, so if you are unsure whether the surface you are looking at is a cleavage plane, try rotating the mineral in bright light. If cleavage is present, you will generally find that all of the cleavage surfaces will glint in the light for a given cleavage direction. Crystal faces will also glint in the light, but they do not repeat themselves at depth throughout the mineral. The best way to overcome these problems is to look at many examples. It’s worth it to be able to identify cleavage and fracture because cleavage is a reliable diagnostic property for most minerals.
Can You Pick Out the Broken Piece?
IMPORTANT: To Receive points for this activity, make sure you advance the slides to the end!
Density
Density is a measure of the mass of a mineral per unit volume, and it is a useful diagnostic tool in some cases. Most common minerals, such as quartz, feldspar, calcite, amphibole, and mica, are of average density (2.6 to 3.0 g/cm3), and it would be difficult to tell them apart based on their density. On the other hand, many of metallic minerals, such as pyrite, hematite, and magnetite, have densities over 5 g/cm3. If you picked up a sample of one of these minerals, it would feel much heavier compared to a similarly sized sample of a mineral with average density. A limitation of using density as a diagnostic tool is that one cannot assess it in minerals that are a small part of rock with other minerals in it.
Other properties
Several other properties are useful for the identification of some minerals. Some of these are:
- Calcite reacts with dilute acid and will give off bubbles of carbon dioxide.
- Magnetite is strongly magnetic, and some other minerals are weakly magnetic.
- Sphalerite ((Zn,Fe)S) gives off a smell of sulfur when drawn across a streak plate.
- Halite tastes salty.
- Talc feels soapy to the touch.
- Plagioclase feldspar has striations (parallel razor-thin lines etched on the surface) and some varieties show a play of colors when light hits them at the right angle (Google the mineral labradorite!)

