ANTIBIOTICS
LEARNING OBJECTIVES
Interpret results of Kirby-Bauer disk diffusion susceptibility tests
Differentiate between the Kirby-Bauer disk diffusion susceptibility test and the E-test
Interpret results of E-tests
Interpret the result of a beta-lactamase test
MCCCD OFFICIAL COURSE COMPTENCIES
Describe and compare the effectiveness of physical and chemical methods of microbial control
Identify structural characteristics of the major groups of microorganism
Compare and contrast prokaryotic cell and eukaryotic cells
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
Apply various laboratory techniques to identify types of microorganisms
MATERIALS
Students will complete the lab in groups of 3 at Pecos and groups of 4 at Williams
Mueller-Hinton agar plates with confluent lawns of:
- Escherichia coli (gram-negative)
- Pseudomonas aeruginosa (gram-negative)
- Staphylococcus aureus (gram-positive)
Antibiotic disks for Kirby-Bauer Disk Diffusion Susceptibility Test:
- Zithromax (brand name) or Azithromycin (generic)
- Augmentin (brandname) or Amoxicillin and Clavulanic Acid (generic)
- Cipro (brand name) or Ciprofloxacin (generic)
- Bactrim (brand name) or Sulfamethoxazole and Trimethoprim (generic)
- Neosporin (brand name, topical) Poly-Rx (brand name, systemic) or Polymyxin B (generic)
E-Test:
- Cipro (brand name) or Ciprofloxacin (generic)
Metric Ruler
BIOCHEMICAL TEST ALBUM
| ANTIBIOTIC TARGET | MECHANISM |
|---|---|
| Bacterial Cell Wall Synthesis | Blocks peptidoglycan synthesis, osmotic forces cause bacteria to shrivel or burst |
| Disrupt Membranes | Disorganize the structure or inhibit the function of membranes |
| Nucleic Acid Synthesis | Block synthesis of DNA or RNA or bind to DNA or RNA so they cannot be replicated, transcribed, or translated |
| Protein synthesis | Target bacterial 70s ribosome |
| Metabolic Pathways | Act as a competitive inhibitor and occupy the active site of an enzyme so the substrate can’t bind |
The range of bacteria killed by an antibiotic determines its “spectrum of activity”. Antibiotics that are only effective against gram-positive or gram-negative bacteria have a narrow spectrum of activity. If the pathogen causing an infection has been identified, it is best to use a narrow-spectrum antibiotic and minimize collateral damage to the normal microbiome.
Antibiotics that are effective against many different types of bacteria including both gram-positive and gram-negative species are called broad spectrum antibiotics. A broad-spectrum antibiotic may be prescribed while waiting on the laboratory identification of the infecting pathogen. Broad-spectrum antibiotics are also used for polymicrobic infections (mixed infection with multiple bacterial species), as prophylactic prevention of infections with surgery or invasive procedures, or to treat an infection when a narrow-spectrum drug fails because of development of drug resistance by the target pathogen.
The risk associated with using broad-spectrum antibiotics is that they will also target the normal microbiome increasing the risk of a superinfection. A superinfection develops when the antibiotic used to treat the infection wipes out a person’s microbiota as well as the pathogen they are intended to kill. Micoorganisms such as Candida albicans and Clostridium difficile grow out of control when they do not have to compete with microbes in the microbiota.
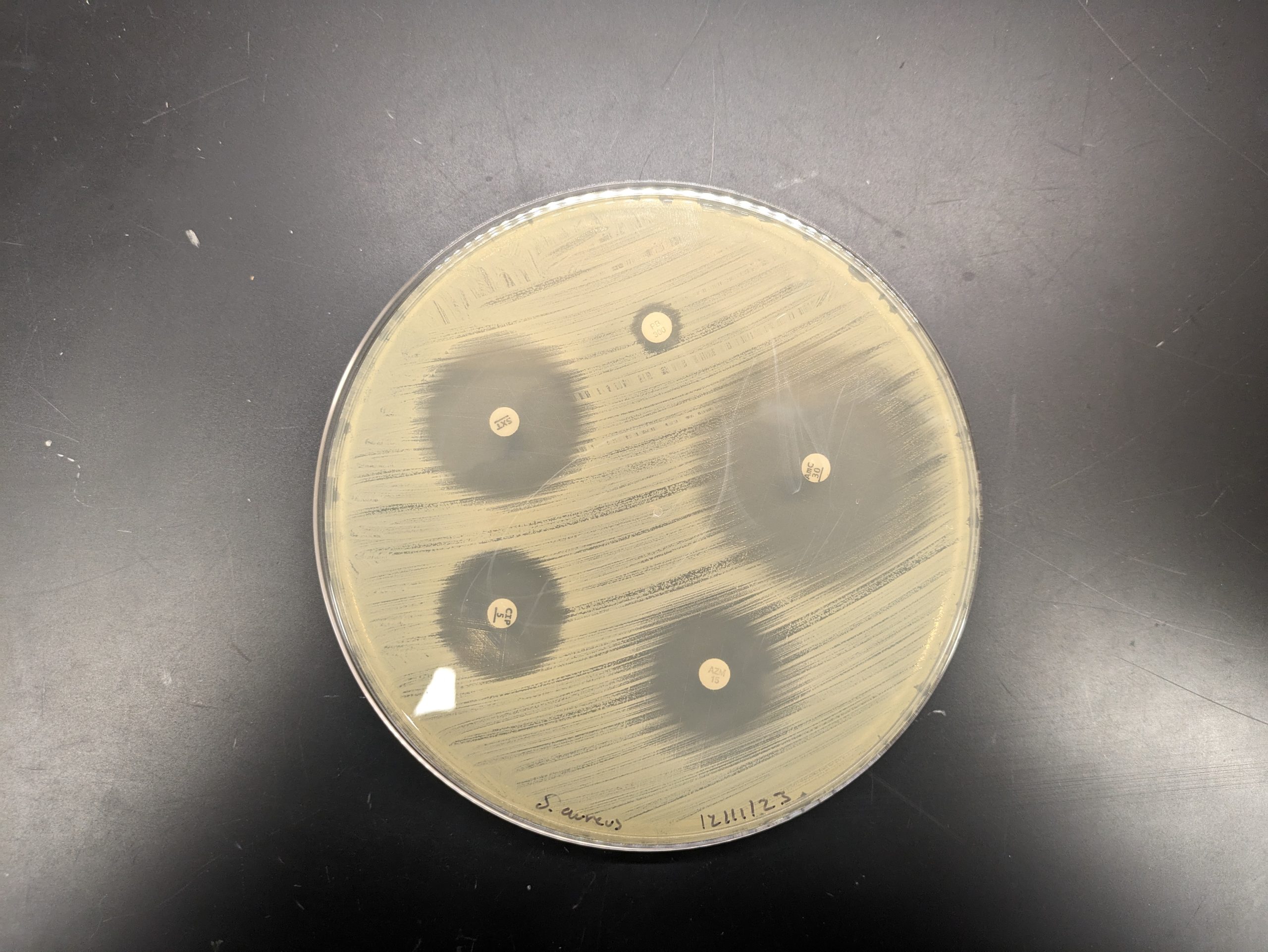
Kirby-Bauer results can be used to determine spectrum of activity of an antibiotic. If only gram-positive or gram-negative bacteria are sensitive to the antibiotic, it is a narrow-spectrum antibiotic. If both gram-positive and gram-negative bacterial species are sensitive to the antibiotic, it is a broad-spectrum antibiotic. Note that a broad-spectrum antibiotic does not have to kill every gram-positive and gram-negative bacteria as some bacteria may be resistant to the antibiotic.
The epsilometer or E-Test combines a disk diffusion susceptibility pattern with the determination of Minimum Inhibitory Concentration (MIC). The MIC test determines the lowest concentration of an antibiotic at which bacterial growth is completely inhibited therefore MIC determines the dosage of the antibiotic that should be effective in controlling the infection in the patient. An antibiotic gradient is present on the E-Test plastic strip.
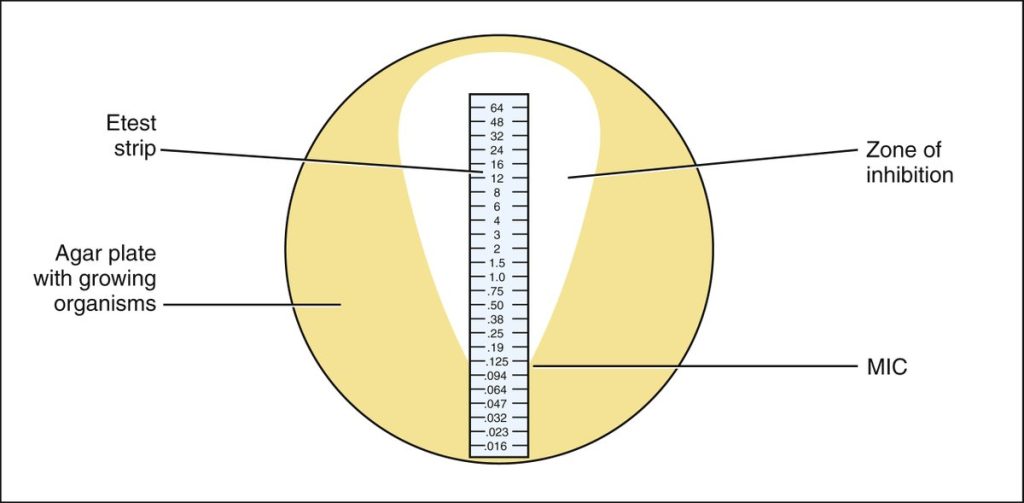
When the E- test strip is applied to an inoculated Muller-Hinton agar plate, there is an immediate release of the antibiotic and the establishment of an antibiotic concentration gradient on the agar. After incubation, bacterial growth becomes visible on the plate, and a symmetrical inhibition ellipse along the strip is seen. The MIC value in ug/mL is read at the point where ellipse intersects the scale of the E-test strip.
The following antibiotics were tested:
| ANTIBIOTIC |
TYPE OF ANTIBIOTIC |
TARGET | USED TO TREAT |
|---|---|---|---|
| Zithromax (brand name)
Azithromycin (generic) |
Semi-synthetic | Protein synthesis | Ear, Skin, Eye, Respiratory, Sinus, and Sexually Transmitted Infections |
| Augmentin (brand name)
Amoxicillin and Clavulanic Acid (generic) |
Semi-synthetic | Amoxicillin – bacterial cell wall synthesis
Clavulanic Acid – inactivates some beta-lactamase enzymes |
Sinus, Ear, Respiratory, Skin, and Urinary Infections |
| Cipro (brand name) Ciprofloxacin (generic) | Synthetic | Nucleic acid synthesis – bacterial DNA gyrase | Urinary, Skin, Bone, Joint, Sexually Transmitted, Respiratory, Gastrointestinal Infections |
| Bactrim (brand name) Sulfamethoxazole and Trimethoprim (generic) | Antibiotic | Metabolic pathways – folic acid synthesis | Minor cuts, scrapes, and burns, Meningitis, Respiratory, Urinary Infections, and Sepsis |
| Neosporin (brand name, topical)
Poly-Rx (brand name, systemic) Polymyxin B (generic, systemic) |
Synthetic | Disrupts membranes | Urinary, Ear, Respiratory, and Gastrointestinal Infections |
Some bacteria produce the beta-lactamase enzyme. Beta-lactamase destroys the beta-lactam ring of penicillin. The end product will be penicilloic acid.
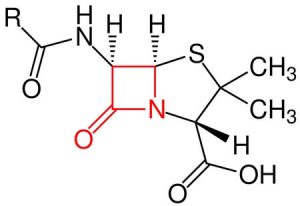

Deionized water is added to a tube containing penicillin and a pH indicator. The solution in the tube will be pink. A heavy inoculum of bacteria is added to the tube. The tube is observed for a color change (pink to yellow).
Yellow = Positive (due to the acidic pH change from production of penicilloic acid)
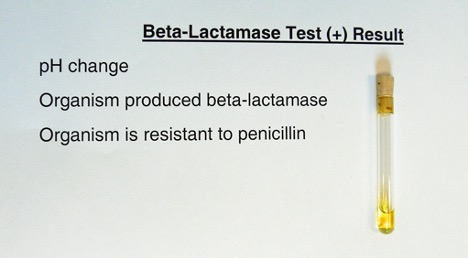
Pink = Negative (no color change because no penicilloic acid was produced)

Complete the beta-lactamase portion of the worksheet.
PRE-ASSESSMENT
procedure
Students will complete the lab in groups of 3 at Pecos and groups of 4 at Williams
INTERPRETING THE KIRBY-BAUER DISK DIFFUSION SUSCEPTIBILITY TEST
Step 1. Measure the diameter of the zone of inhibition from one edge to the other; right over the middle of the antibiotic disk. If the bacteria grow right up to the disk, the zone of inhibition is reported as “0 mm.” This indicates that the bacteria are resistant to that antibiotic. If mutant strains are growing inside the general zone of inhibition or if the edges of the zones are “fuzzy”, measure the innermost clear zone. Record the results on the worksheet.
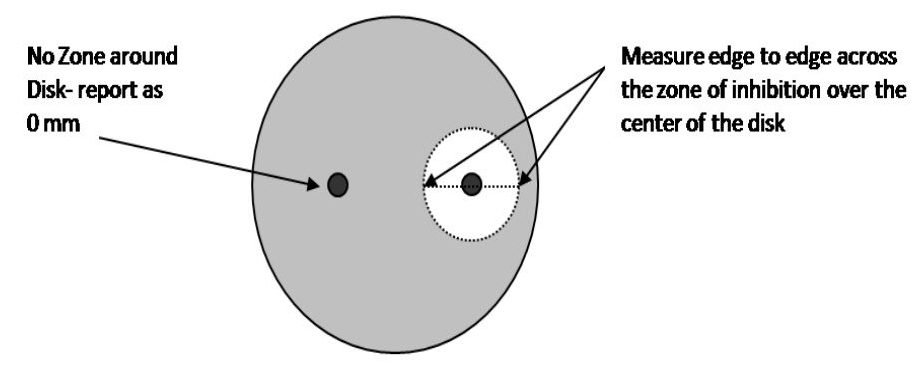
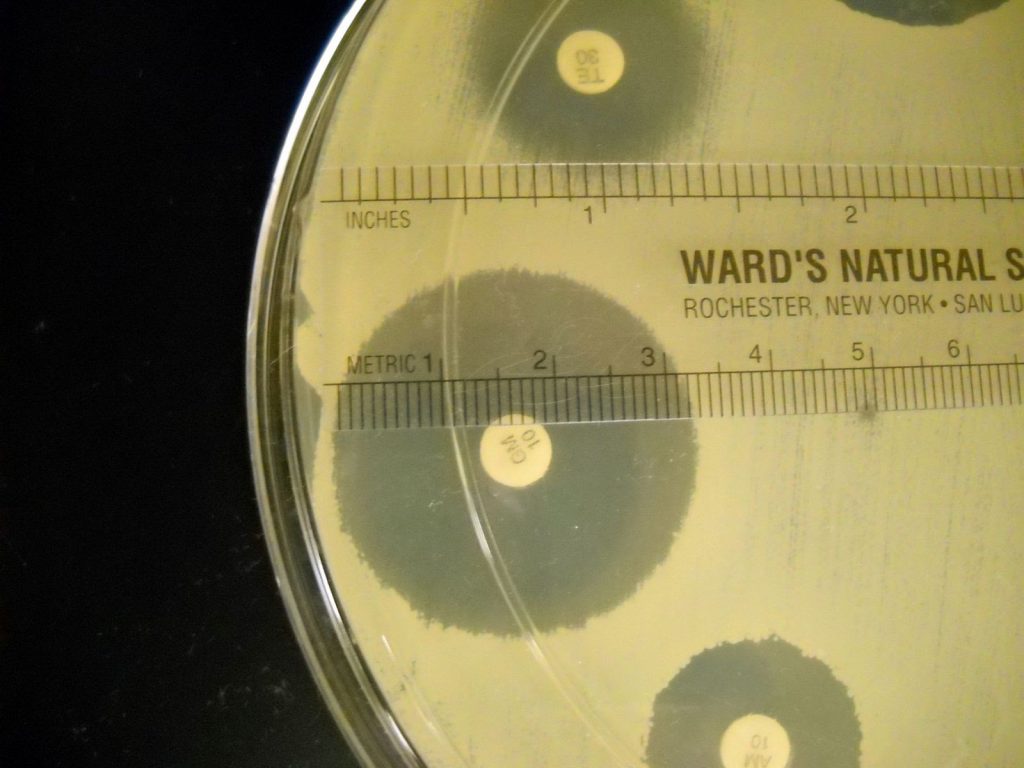
Step 2. The zone of inhibition is the result of two factors: how quickly the bacteria grows and how fast the antibiotic diffuses through the media. Since antibiotics have different molecular weight and therefore different diffusion rates, antibiotics cannot be compared to each other. Instead, each antibiotic zone size must be compared to a standardized chart. Compare the zone sizes of your zones to the standardized chart on the worksheet. Determine If the bacteria is sensitive, intermediate, or resistant to each antibiotic. Record your results on the worksheet.
Step 3. Using the S, I, R results for the tested bacteria, complete the spectrum of activity chart in the worksheet.
E-TEST TO DETERMINE MINIMUM INHIBITORY CONCENTRATION (MIC)
Step 1. Determine the MIC for the E-Test plates results shown on the worksheet. The MIC value is read from the scale in terms of µg/mL where the ellipse edge intersects the strip. Examples of E- Test results and interpretation are shown below.
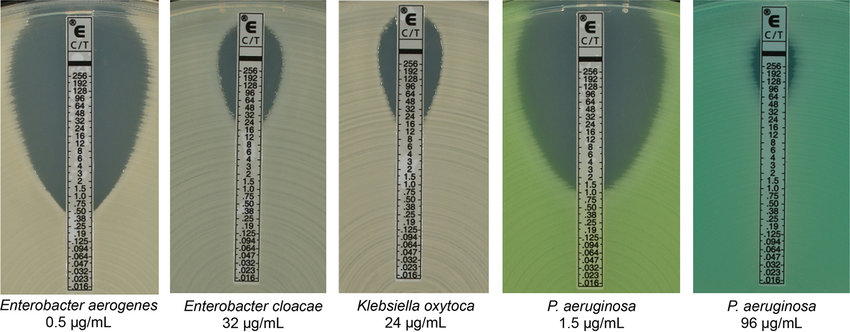
Step 2. Record the MIC results in the worksheet.
Step 3. Answer the beta-lactamase test questions in the worksheet.
POST TEST
DISCOVERIES IN MICROBIOLOGY

DR. JANE HINTON
American veterinarian Dr. Jane Hinton helped create Muller Hinton agar in 1941. It is non-selective, non-differential medium, so almost all bacteria can grow on it. The starch in the media absorbs toxins released from the bacteria, so they do not interfere with the antibiotic. It is a loose agar; this allows for better diffusion of the antibiotics than most other plates. A better diffusion leads to a more accurate zone of inhibition.

