ACID-FAST STAIN
LEARNING OBJECTIVES
Perform an acid-fast stain
Distinguish acid fast from non-acid-fast organisms
Explain why some bacterial cell walls cannot be stained with traditional Gram stain
Discuss how the acid-fast can be used to identify microorganisms and why it is an important tool in clinical settings
MCCCD OFFICIAL COURSE COMPETENCIES
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cells
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
Utilize aseptic technique for safe handling of microorganisms
Apply various laboratory techniques to identify types of microorganisms
MATERIALS
Stock cultures:
TSA slant of Mycobacterium smegmatis
TSB broth of Staphylococcus epidermidis
Equipment:
1 glass microscope slide per person
Inoculating loop
Microscope
Stains:
Carbolfuchsin (primary stain)
Acid-alcohol (decolorizer)
Methylene blue (secondary or counterstain)
BACTERIA ALBUM LINK
The acid-fast stain was developed in 1882 by Paul Ehrlich to aid in the diagnosis of tuberculosis (TB). Ehrlich observed that dyes were not absorbed by Mycobacterium unless the organism on the slide was heated. Once the dye was absorbed, the cell wall retained the dye even when the smear was washed with a mixture of acid and alcohol (3% hydrochloric acid and 95% ethanol).
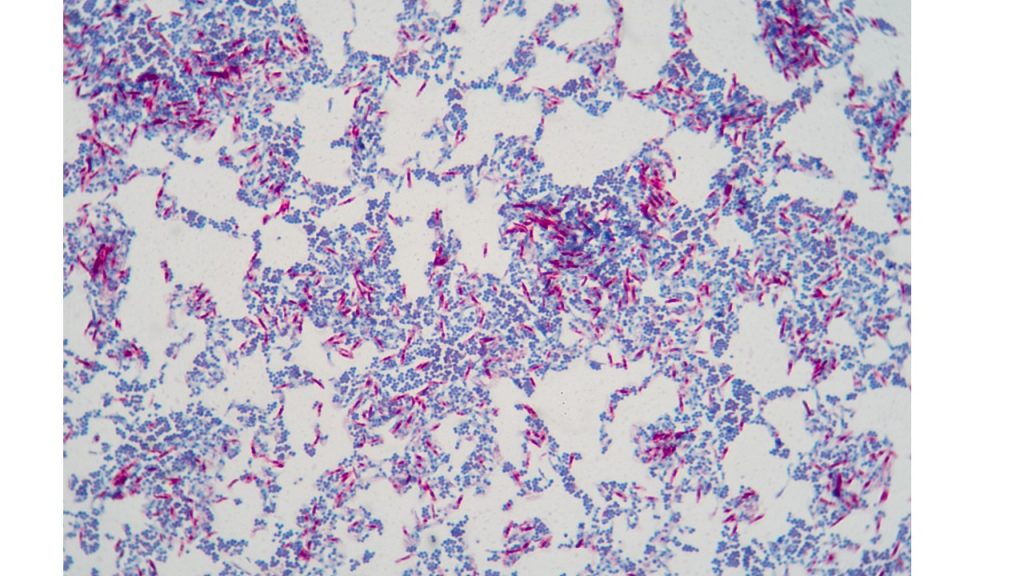
The cell wall of bacteria Mycobacterium, Nocardia and parasite Cryptosporidium contains large amounts of glycolipids, especially mycolic acids. This unique cell wall enables them to resist staining and makes them resistant to many common disinfectants. It is the mycolic acid that makes it difficult to absorb dye into the cell. However, once the dye penetrates the cell wall (by using heat or chemicals such as phenol), these glycolipids prevent acid-alcohol from decolorizing the cell. Therefore, these organisms are said to be “acid-fast”. Most bacteria, such as E. coli and Staphylococcus, lack this high mycolic acid content and are easily decolorized by the acid-alcohol. Therefore, these organisms are described as “non-acid-fast”. They absorb the counterstain, methylene blue. The acid-fast stain is therefore a differential stain since it uses two dyes to distinguish two groups of organisms based on the mycolic acid in their cell walls.
There are two methods of acid-fast staining. The Ziehl-Neelsen stain uses steam to penetrate the mycolic acid in the cell walls, whereas, Kinyoun uses a very concentrated dye, hard heat fixing, and longer exposure time.
Several species of Mycobacterium and Nocardia are pathogenic for animals and humans. Mycobacterium tuberculosis causes TB and is one of the world’s deadliest diseases. According to the CDC, it is estimated that 23% of the global population has latent tuberculosis infections. 1 in 10 will go on to develop the disease. Globally, there are approximately 10 million TB infections per year. There are approximately 1.5 million TB-related deaths worldwide. This disease is the number one cause of death world-wide in patients who have AIDS.
Mycobacterium leprae causes leprosy or Hansen’s disease. Leprosy is rare in the United States with only 150-250 cases per year. In the southern United States, some armadillos are naturally infected with the bacteria that cause Hansen’s disease in people and it may be possible that they can spread it to people. However, the risk is very low and most people who have contact with armadillos are unlikely to get Hansen’s disease.
Worldwide, according to WHO, there are approximately 200,000 new cases of leprosy per year worldwide. India accounts for more than 50% of the global cases. The National Hansen’s Disease Program in Baton Rouge, Louisiana, is the only institution in the United States exclusively devoted to Hansen’s disease. The center functions as a referral and consulting center with related research and training activities. Most American patients are treated under U.S. Public Health Service grants at clinics in major cities or by private physicians.
Nocardia asteroides causes a pulmonary disease called nocardiosis which can resemble tuberculosis. In the United States, around 500-1,000 new cases of Nocardia infection occur annually. An estimated 10-15% of these patients also have HIV infection. Nocardia can be differentiated from Mycobacterium when stained since Nocardia is usually a branching, filamentous organism and Mycobacterium is a rod-shaped organism.
Cryptosporidium, a protozoan parasite, invades the epithelial cells lining the human digestive tract causing a diarrheal disease called cryptosporidiosis. While this parasite can be transmitted in several different ways, water is a common method of transmission and Cryptosporidium is one of the most frequent causes of waterborne disease among humans in the United States. Diagnosis is achieved through finding oocysts in stool specimens from infected patients. The oocysts are difficult to see with routine stains so a modified acid-fast stain can be used in which stained oocysts appear bright red against a blue-green background.
PRE-ASSESSMENT
PROCEDURE
Prepare a mixed culture smear for Kinyoun Acid-Fast Stain
For this Exercise: Use a TSA slant of Mycobacterium and TSB broth of Staphylococcus
1. Clean a glass slide with lens cleaner and a cotton swab. Dispose of the cotton swab in the regular trash. Using a permanent marker, label the top right corner of one slide with “A” for Acid-fast.
2. Sterilize an inoculating loop and allow it to cool.
3. Remove the cap of the Staphylococcus broth, insert the inoculating loop and obtain 2 to 3 loopfuls of inoculum.
4. Gently spread the inoculum in the circle. Then, sterilize the loop and let it cool.
5. Remove the cap from the Mycobacterium, insert the inoculating loop and obtain tiny amount of inoculum.
6. Mix the tiny amount of Mycobacterium in the drop of Staphylococcus. Mash the organism against the slide to mix it.
7. Dry the slide on the slide warmer.
Acid fast staining procedure
1. Begin the procedure with the dried slide on the slide warmer. Put a small piece of filter paper on the slide and add carbolfuchsin to the paper.
2. Keep adding more carbolfuchsin when the paper looks dry or the edges of the stained paper look gold.
3. After 2 minutes, remove the filter paper from the slide; place it on a paper towel and dispose of it in the trash.
4. Transfer the slide to the stain rack.
5. Rinse the slide thoroughly with deionized water. Drain off excess water.
6. Decolorize the smear by adding acid-alcohol to the slide for 5 seconds.
7. Rinse the slide with deionized water. Drain off the excess water.
8. Add Loeffler’s methylene blue stain to the slide. Allow the dye to sit on the slide for one minute.
9. Rinse with deionized water. Blot dry with bibulous paper or paper towel.
10. Observe the smear under oil immersion. Draw your observations on the worksheet and label an example of the acid fast and non-acid fast organisms.
11. After you have completed your observation the acid-fast stain, dispose of the slide in the used slide basin.
POST TEST
DISCOVERIES IN MICROBIOLOGY

ELIZABETH BUGIE GREGORY
Elizabeth Bugie Gregory was an American microbiologist and biochemist. In 1944, Gregory, Selman Waksman, and Albert Schatz discovered the antibiotic streptomycin. Gregory was told that it was not important for her name to be on the patent as she would “one day get married and have a family”. Waksman went on the win the Nobel Prize for medicine in 1952 and took full credit for the discovery. Through a court settlement, Schatz was awarded 3% of the royalties for streptomycin and was officially recognized as co-discoverer of streptomycin. Waksman claimed that Gregory was more involved in the discovery than Schatz. Gregory was awarded 0.2% of the royalties for streptomycin.

