FUNGI
LEARNING OBJECTIVES
Differentiate between fungi and bacteria
Differentiate between yeast and mold
Perform basic mycological culturing and staining procedures
Identify yeast and molds based on their macroscopic and microscopic characteristics
MCCCD OFFICIAL COURSE COMPETENCIES
Identify structural characteristics of the major groups of microorganism
Compare and contrast prokaryotic cell and eukaryotic cells
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Utilize aseptic technique for safe handling of microorganisms
MATERIALS
Students will complete the lab in groups of 3 at Pecos and groups of 4 at Williams
Cultures:
1 Yeast (Saccharomyces cerevisiae) on Sabouraud’s dextrose agar. The culture will be used for two lab days.
Select one of the three unknown mold cultures on Sabouraud’s dextrose agar slants. The cultures will be used for two lab days.
Media:
1 Sabouraud dextrose agar plate/group
Equipment:
Methylene blue stain
Dissecting needle
Inoculating loop
Microscope slides
Cover slips
1 wooden applicator stick
1 empty Petri dish
1 piece of filter paper
Deionized water in a dropper bottle
Microscope
Lactophenol cotton blue stain
Demonstration slides of mold: Rhizopus, Aspergillus, and Penicillium
FUNGI ALBUM LINK
Mycology is the study of fungus. Fungi are eukaryotic organisms which grow as either yeast or mold. However, there are some fungi that are dimorphic, meaning they can grow as yeast under certain environmental conditions (such as the warm moist lungs in the body) and mold under other conditions (such as in soil in the environment). Coccidioides immitis, the fungus responsible for San Joaquin Valley Fever, is an example of a dimorphic fungus.
Fungi grow slower than bacteria and at a lower temperature and lower pH than most bacteria prefer. Sabouraud agar is selective media for fungi because it incorporates simple nutrients (glucose and peptone) at a pH of 4.5-5.6 which inhibits bacterial growth. Although some yeasts can grow at 36°C, we incubate all fungal cultures at 25 to 30°C for at least one week. Some fungi take three weeks or more to grow.
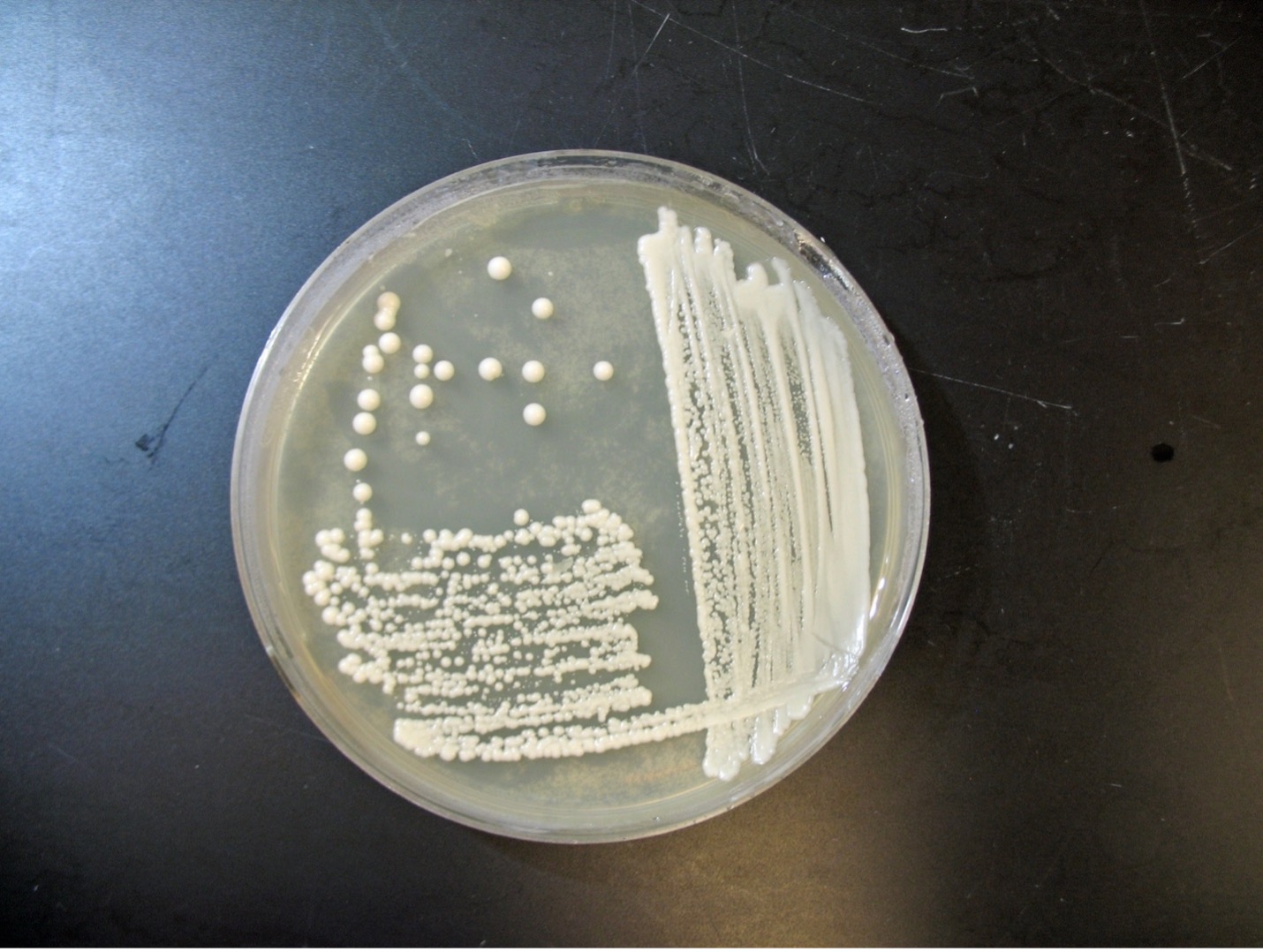
Yeast
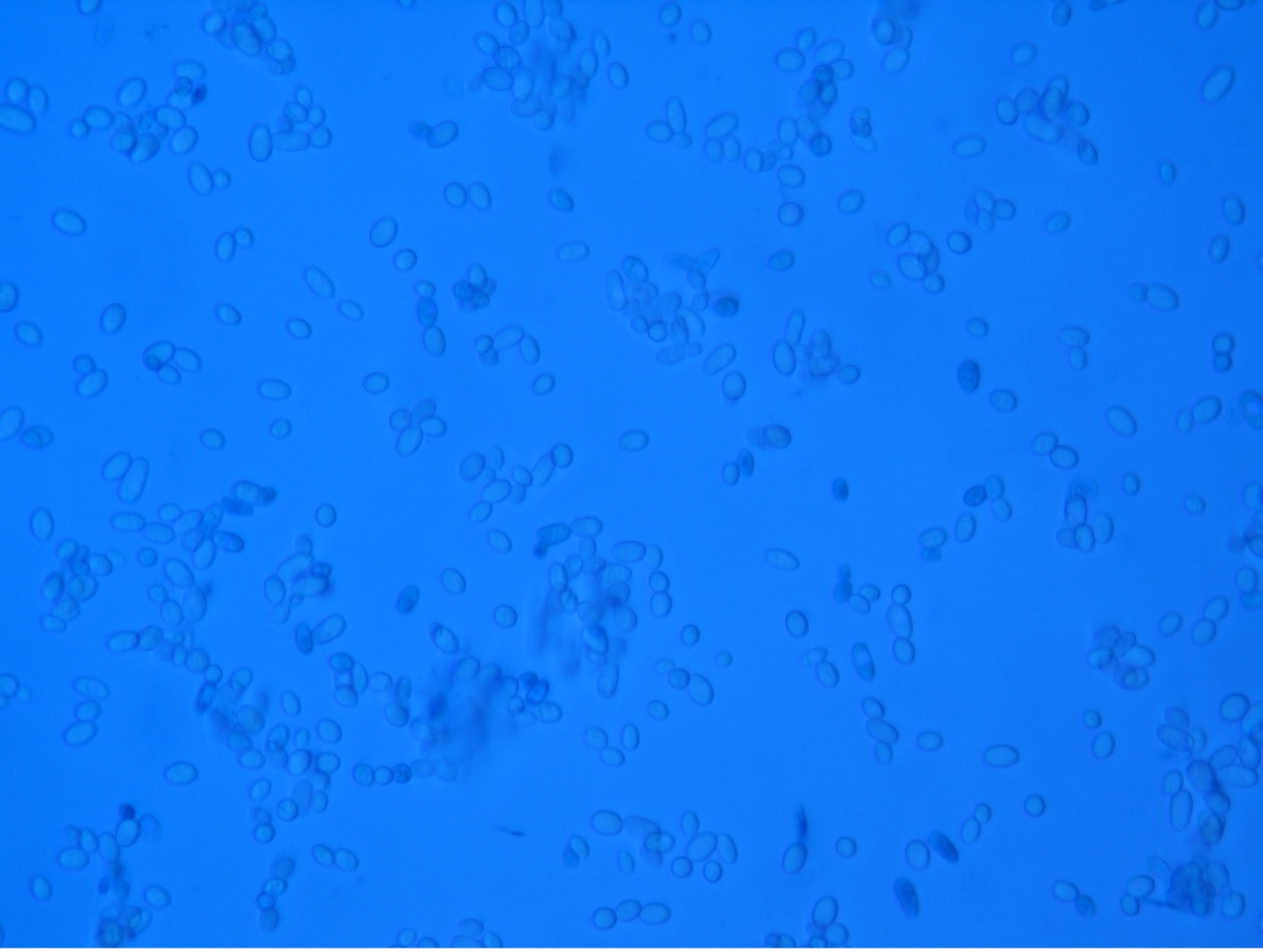
The colonial appearance of most yeast is moist, creamy and white in color and similar in appearance to Staphylococcus colonies. Microscopically, yeast cells are unicellular and round to oval, whereas bacteria cells vary in shape (cocci, bacilli, spirals). Yeast cells are usually five to ten times larger than bacteria and can be visualized at 400X total magnification. Yeast reproduce asexually by budding and the newly produced cell, called a bud or blastospore, protrudes from the periphery of the parent cell. The blastospore may break off from the parent cell or stay attached. Successive blastospores remaining attached to the original cell result in the formation of pseudohyphae.
Most yeast have similar macroscopic and microscopic appearances. Biochemical tests, such as carbohydrate assimilation tests, must be performed to speciate yeast. Examples of yeast include Candida albicans which is an opportunistic pathogen and Saccharomyces cerevisiae which is used to make bread.
Mold
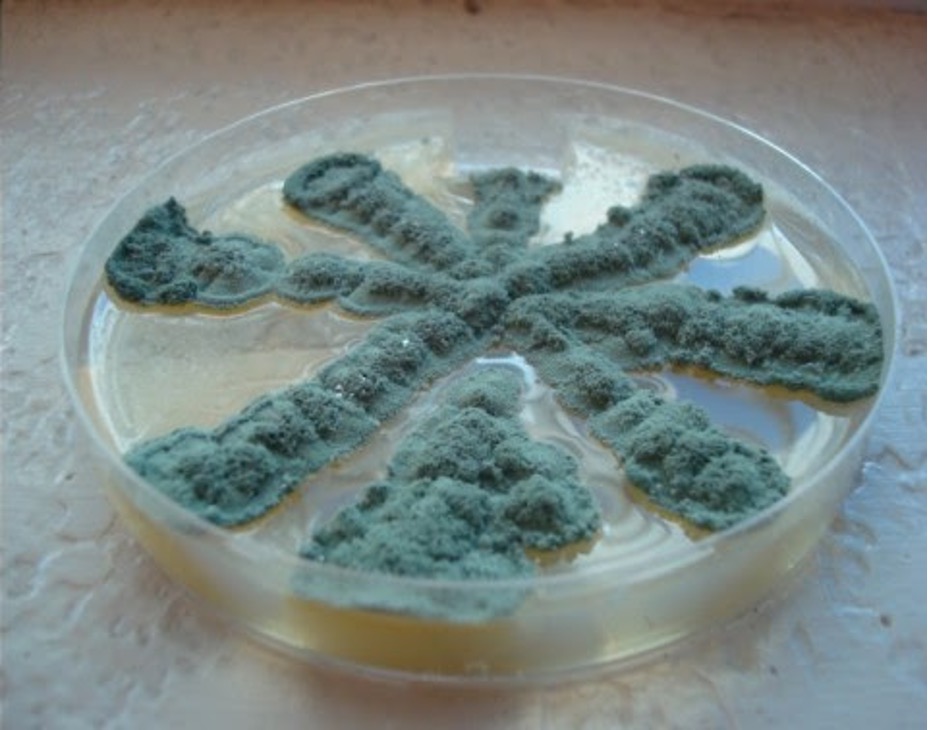
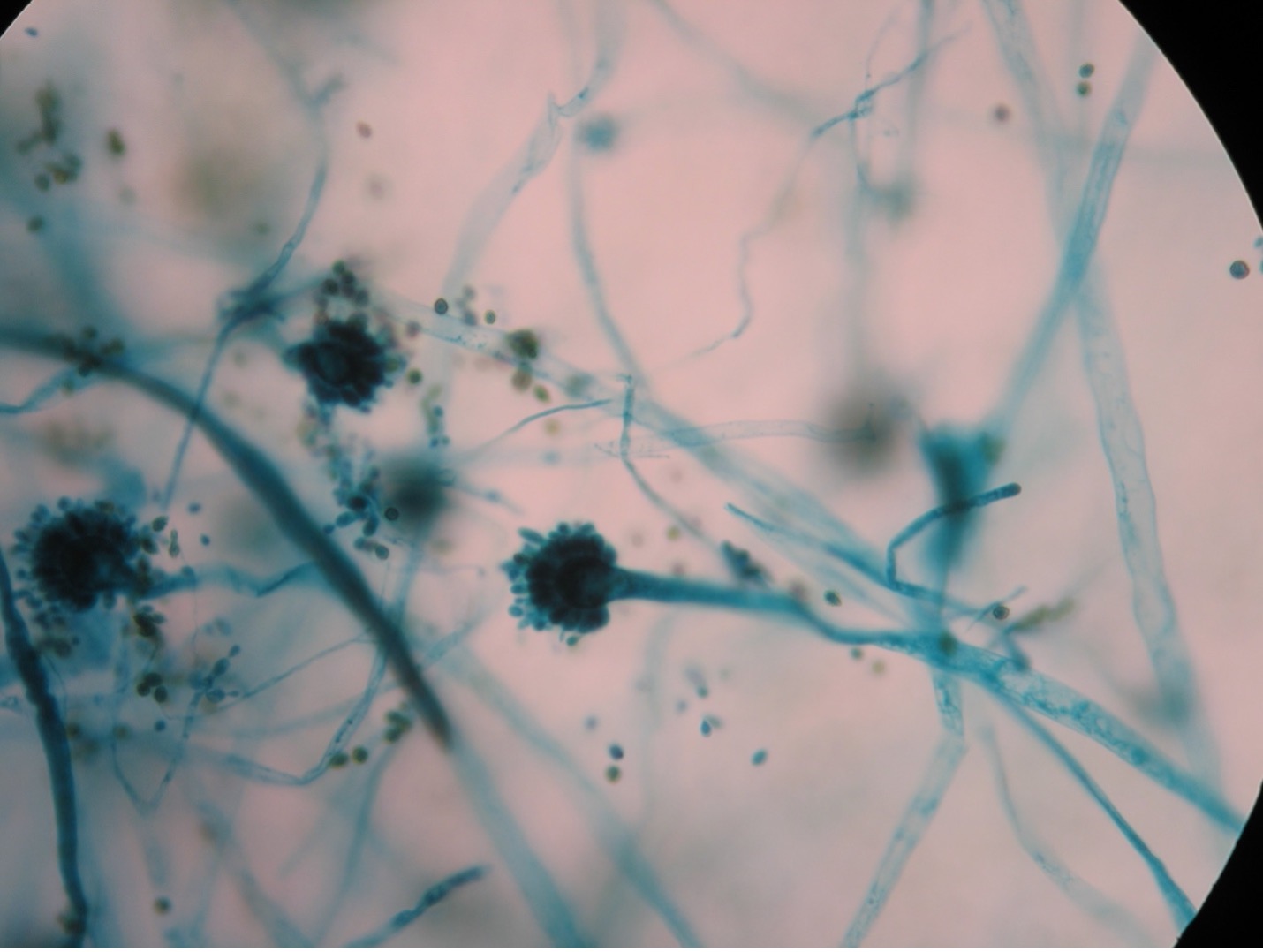
Fungi that grow as mold produce multicellular filaments called hyphae. Most fungal hyphal filaments are separated by a cross wall and are called septate hyphae. The hyphal filaments not separated by cross walls are called aseptate or coencocytic hyphae. Hyphal filaments intertwined into a mass, known as mycelia, can be seen macroscopically as fuzzy or hairy, colorful colonies. Some of the hyphae, called vegetative hyphae, grow on or down into the agar surface to extract nutrients from the medium. Other hyphae, called aerial hyphae, grow above the agar surface and produce asexual reproductive spores.
The two types of asexual spores produced by molds are called sporangiospores and conidiospores. Sporangiospores are produced at the end of aerial hyphae called sporangiophores in a saclike structure called a sporangium. The sporangia of specific molds have characteristic shapes which can be used to identify the mold. An example of a mold that produces sporangiospores is the bread mold, Rhizopus. Conidiospores are formed on aerial hyphae called conidiophores. Conidia may be one-celled (microconidia) or multicelled (macroconidia). Examples of fungi that produce conidiospores are Penicillium and Aspergillus.
Molds are usually identified in the laboratory by their characteristic macroscopic appearance, hyphal structure (septate or nonseptate) and type of asexual sporulation. Since spore formation is an important identification criterion, slide cultures are performed to observe sporulation without disturbing the hyphal structures.
PRE-ASSESSMENT
PROCEDURE
Students will complete the lab in groups of 3 at Pecos and groups of 4 at Williams
DAY 1: Select one of the three unknown molds to culture
Prepare a Mold Slide Culture
1. Obtain a piece of filter paper, an empty Petri dish, a Sabouraud dextrose agar plate, a glass slide, cover slip, dissecting needle, inoculating loop, wooden applicator sticks and an unknown mold culture.
2. Place the piece of filter paper in the empty Petri dish, tearing it into pieces if necessary to get it to fit. Add drops of water until the filter paper is fully saturated.
3. Break a wooden applicator stick in half and place the pieces on top of the filter paper.
4. Place a glass slide on top of the sticks.
5. Sterilize the dissecting needle in a bacticinerator and cut a 1.5 cm block of Sabouraud dextrose Agar (almost the size of the cover slip).
6. Scoop the agar block out of the plate, balancing it on a sterilized inoculating loop and using your dissecting needle to hold it in place. Put the agar block on the top of the glass slide balanced on the applicator sticks.
7. Sterilize the dissecting needle in the bacticinerator and allow it to cool completely.
8. Using the sterile dissecting needle, pick up spores by scraping the needle just inside the outer edge of the mold colony.
9. Transfer the spores to each side of the agar block by touching the dissecting needle to the edge of each side.
10. Once all four sides have been inoculated with the mold, add a cover slip to the top of the agar block and gently press down to make sure the cover slip is in contact with the agar surface.
11. Cover the Petri dish with the lid and label the lid with your name. Incubate right side up in the class plate tray until the next lab session.
Subculture the Mold
1. Label the bottom of the same Petri plate that you used to obtain the agar block for the slide culture.
2. Using a sterilized dissecting needle, pick up mold spores by scraping the needle just inside the outer edge of the unknown mold colony.
3. Transfer those spores to the Sabouraud dextrose agar plate by barely scratching the needle onto the surface of the agar to make a small “star” (*) pattern.
4. Replace the Petri dish lid. Incubate in the class plate tray until the next lab session.
Day 2:
Students will complete the lab in groups of 3 at Pecos and groups of 4 at Williams
Observe Yeast Cells (Microscopic Appearance)
1. Obtain a glass slide, cover slip, methylene blue dye, inoculating loop and a culture of Saccharomyces cerevisiae (baker’s yeast).
2. Place a small drop of methylene blue stain on the clean microscope slide.
3. Using a sterile loop, obtain a small inoculum of yeast from the culture and mix it into the methylene blue stain.
4. Place a cover slip on top of the mixture on the slide. Try to avoid air bubbles.
5. Sketch your observations at 400X total magnification on the worksheet.
Observe Yeast Colonies (Macroscopic Appearance)
1. Observe the colonies of yeast growing on Sabouraud dextrose agar.
2. Record your observations on the worksheet.
3. Return the plate of yeast. Do not discard it.
Identify the Unknown Mold Culture (Macroscopic Appearance)
1. Use the mold culture that grew on the plate you inoculated with a star pattern to identify the macroscopic appearance of your unknown mold.
2. Describe the color of the unknown mold colony from the top (surface pigment) and bottom (reverse pigment) of the plate. Record your observations on the worksheet.
3. Describe the height of the aerial hyphae by using one of the following terms:
Cottony – very high aerial hyphae
Velvety – medium high aerial hyphae
Wooly – medium to low aerial hyphae
Record your observations on the worksheet.
4. Tape the plate shut and dispose of it in the biohazard trash.
Observe the Unknown Mold Culture (Microscopic Appearance)
1. Use the mold culture that grew on the plate with the slide, cover slip, and agar brick set up to identify the microscopic appearance of your unknown mold.
2. Using a sterilized loop or sterilized dissecting needle to gently dislodge the cover slip from the agar block. Place the cover slip on the lab bench.
3. Using a sterilized loop or sterilized dissecting needle gently remove the agar block from the glass slide by tilting the slide and dislodging the agar block. Place the slide on the lab bench.
4. Leave the agar block and wooden applicator sticks in the Petri dish. Tape the lid and dispose of the Petri dish in the biohazard trash.
5. You will now prepare a wet mount of the slide the agar block was sitting on and cover slip that was on top of the agar block. Put a drop of lactophenol cotton blue stain on the slide. Add the cover slip. Try to avoid air bubbles.
6. Examine the wet mount under the microscope at 40X and 100X total magnification. Look for spores that are STILL ATTACHED to the hyphae. Once an area is in view, view the wet mount under 400X total magnification. Sketch your observations on the worksheet.
7. Dispose of your mold wet mount slide in the used slide basin.
Macroscopic and Microscopic Appearance of All Three Unknown Molds
1. You selected one of the three unknown molds options on Day One of this lab exercise. You must be able to identify all three molds based on their macroscopic and microscopic appearance. Observe the prepared slide demonstrations and plate cultures of Aspergillus, Penicillium and Rhizopus. Sketch your observations on the worksheet. Use the pictures below as a guide.


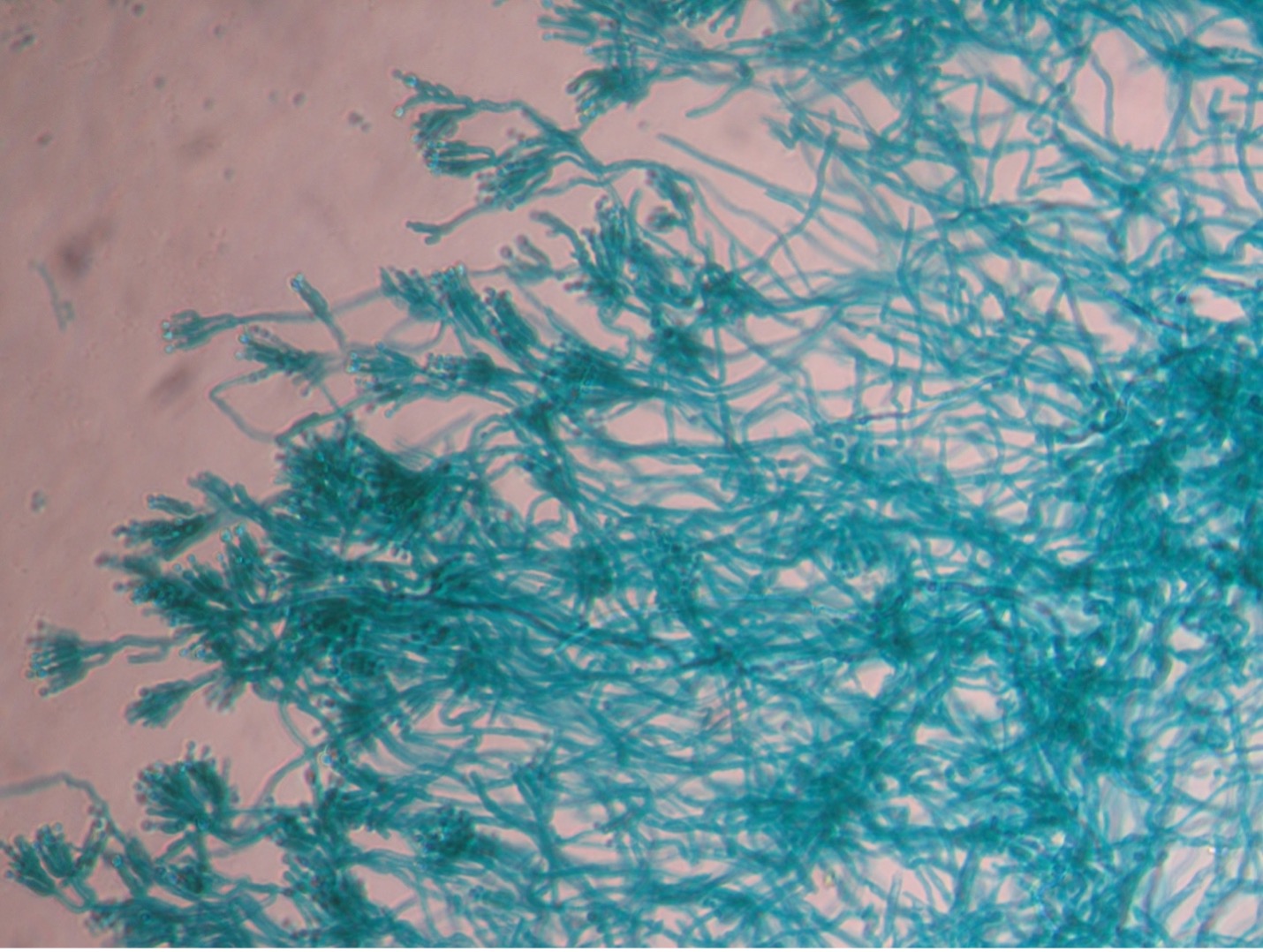
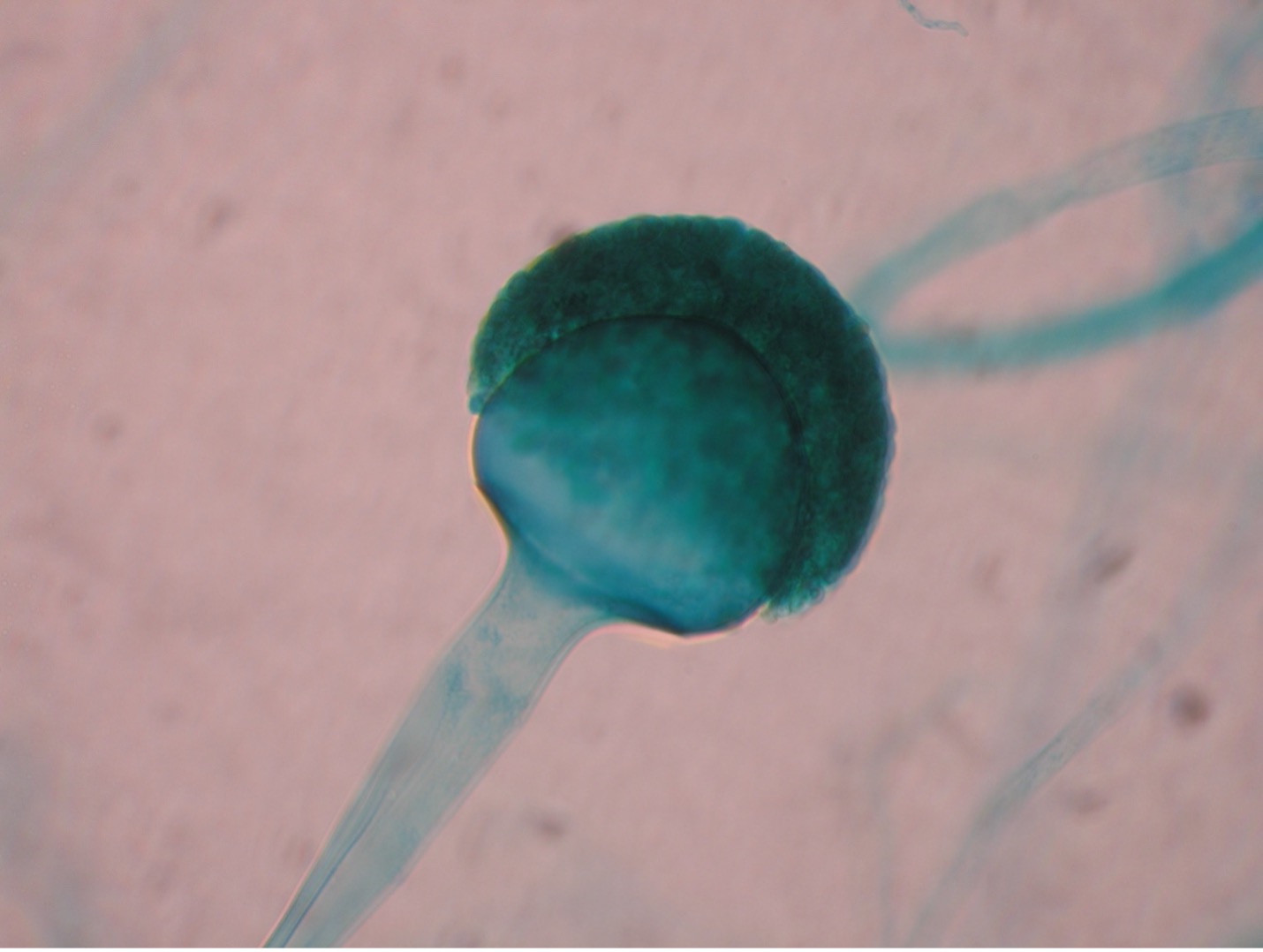
POST TEST
DISCOVERIES IN MICROBIOLOGY

ELIZABETH HAZEN AND FULLER BROWN
In 1950, American chemists Elizabeth Hazen and Fuller Brown discovered the antifungal drug nystatin. Hazen (in New York City) cultured hundreds of soil samples from around the world and tested them in vitro for activity against two fungi (Candida albicans and Cryptococcus neoformans). If the culture killed the two test fungi, she would mail the culture in a mason jar to Brown (in Albany New York). Brown isolated the active agent in the culture and would send it back to Hazen. Hazen would then test it again against the two test fungi. If the active agent from the culture killed the two test fungi, its toxicity was evaluated in animals. Nearly all the agents that killed the test fungi turned out to be highly toxic in animals. Hazen and Brown tested hundreds of soil samples from around the world. The one successful culture was found in soil from the garden of a friend of Hazen. The culture contained an active agent they named nystatin (for New York State). Nystatin has been used for years as an effective treatment for fungal infections of the skin, mouth, vagina, and intestinal tract.

