ANTISEPTICS AND DISINFECTANTS
LEARNING OBJECTIVES
Explain the difference between a disinfectant and antiseptic
Evaluate the results of disk diffusion sensitivity testing of antiseptics and disinfectants
MCCCD OFFICIAL COURSE COMPTENCIES
Describe and compare the effectiveness of physical and chemical methods of microbial control
Identify structural characteristics of the major groups of microorganism
Compare and contrast prokaryotic cell and eukaryotic cells
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Utilize aseptic technique for safe handling of microorganisms
MATERIALS
Students will complete the lab in groups of 3 at Pecos and groups of 4 at Williams
TSA plates with confluent lawns of:
- Escherichia coli (Gram negative)
- Pseudomonas aeruginosa (Gram negative)
- Staphylococcus aureus (Gram positive)
Antiseptic and Disinfectant Disks:
- Hydrogen peroxide
- Isopropyl alcohol
- Oregano oil
- Clorox clean-up multi-surface cleaner with bleach
- Clorox free and clear multi surface cleaner
- Microban multipurpose cleaner
- Windex multipurpose disinfectant cleaner
- 7th generation all purpose cleaner
There are many products on the market today that claim to kill microorganisms. The Lysol disinfecting wipes label states to disinfect a surface you must – Pre-clean surface. Use enough fresh wipes to thoroughly wet surface. Allow to remain wet for 4 minutes. Allow surface to air dry. Toss dirty wipe away.
Do consumers use enough wipes for the surface to remain wet for 4 minutes?

The Lysol disinfecting wipes label states – Lysol Disinfecting Wipes eliminate the following bacteria and viruses from hard, non-porous surfaces when used as directed: Herpes Simplex Virus Type 1, Human Coronavirus, Influenza A Virus (H1N1), Respiratory Syncytial Virus, Streptococcus pyogenes, Staphylococcus aureus, Escherichia coli O157:H7, methicillin resistant Staphylococcus aureus, and Salmonella enterica.
Are 4 viruses and 5 bacteria 99.9% of viruses and bacteria?
Washing our hands, taking regular showers or baths, and brushing our teeth with toothpaste are common practices we use to control microbial growth on our bodies. In our homes, we try to control the growth of microorganisms by cooking and refrigerating food, cleaning our kitchen and bathrooms with chemicals, and washing our dishes and clothes with detergents.
Sterilization is the destruction of all forms of microbes. Sterilization eliminates all pathogens. Sterilization can be easily accomplished with the use of an autoclave or incineration, but rarely do chemicals available in the marketplace sterilize. Surgeons use sterile instruments when performing surgery. An unopened can of food is sterile inside; during the canning process the contents of the can are sterilized.
When you are practicing personal hygiene or cleaning your house you are not sterilizing, you are disinfecting. Disinfection is use of physical or chemical agent (“disinfectant”) to inhibit or destroy microbes on inanimate objects or surfaces. Disinfection does not eliminate all pathogens and does not kill all microorganisms.
Antisepsis is disinfection of tissue via a chemical agent (“antiseptic”) safe to use on living tissue. Since antisepsis is a type of disinfection it does not eliminate all pathogens and does not kill all microorganisms. Some chemicals can be used as both a disinfectant and an antiseptic depending on their concentration.
Alcohol is used as an antiseptic before a blood draw or administration of an injection. Alcohol is the active ingredient in most hand sanitizers. Alcohol damages the membranes of microorganisms by dissolving their membranes and denatures proteins.
Bleach (sodium hypochlorite) has been used as a disinfectant for over 200 years. Bleach is the active ingredient in nearly one third of all antimicrobial chemicals currently on the market. Bleach denatures the proteins of microorganisms.
Hydrogen peroxide is used as an antiseptic. Hydrogen peroxide generates free radicals that damage microorganisms.
Oregano oil exhibits antimicrobial properties, including antibacterial, antifungal, and antiviral effects, due to the presence of compounds like carvacrol and thymol. Carvacrol disrupts bacterial cell membranes and inhibits biofilm formation. Thymol is a phenolic compound that damages cell walls and cell membranes, interferes with fungal cell membrane synthesis, generates free radicals that damage microorganisms, and interferes with DNA and RNA synthesis.

Clorox clean-up multi-surface cleaner with bleach contains sodium hypochlorite as the active ingredient. The label states when used as directed it kills Escherichia coli, Staphylococcus aureus, Salmonella enterica, rhinovirus, influenza virus A2, Aspergillus niger, Trichophytan interdigitale.
To disinfect non-porous surfaces spray 6-8 inches away from surface. Surface must remain visibly wet for 30 seconds.
To kill mold and mildew spray 4-6 inches from surface until thoroughly wet. Surface must remain visibly wet for 5 minutes.

Clorox free and clear multi-surface cleaner contains ethanol as the active ingredient. The cleaner also contains surfactants and sodium hydroxide for an alkaline pH. The label does not list any microorganisms that it kills.
The directions for use-spray soiled area and wipe with paper towel or cloth.

The active ingredients in Microban 24-hour multi-purpose cleaner are a group of quaternary ammonium compounds, also known as “quats”. Quats are a class of disinfectants that are commonly used in household cleaners. They work by disrupting the cell membranes of microorganisms. The label states if used as directed it it kills Pseudomonas aeruginosa, Salmonella enterica, Staphylococcus aureus, Escherichia coli 0157:H7, human coronavirus 229E, herpes simplex virus type 1, herpes simplex virus type 2, influenza virus AH1N1, respiratory syncytial virus, SARS related coronavirus 2, rotavirus, Trichophytan interdigitale, Aspergillus niger, Penicillium.
To disinfect hard, non-porous surface spray 6-8 inches until thoroughly wet. Let stand 5 minutes.

The active ingredient in Windex multipurpose cleaner is lactic acid. Lactic acid lowers pH, disrupts membranes, and denatures proteins. The label states when used as directed it kills Staphylococcus aureus, Salmonella enterica, Pseudomonas aeruginosa, Streptococcus pyogenes, rhinovirus type 37, influenza A2 Hong Kong, rhinovirus type 37, influenza A2 Hong Kong, influenza B, Enterobacter aerogenes, Escherichia coli, Campylobacter jejuni, Listeria monocytogenes, Streptococcus pyogenes.
To disinfect hard, non-porous surfaces preclean visibly soiled areas. surface spray 6-8 inches until thoroughly wet. Let stand 10 minutes.

7th generation all purpose cleaner contains plant derived surfactants and a plant derived pH adjuster (alkaline) as the active ingredients. The label does not list any microorganisms that it kills.
The directions for use spray entire surface, rinse and wipe clean.
The purpose of this lab is to test the effectiveness of the antiseptics and disinfectants.
PRE-ASSESSMENT
Students will complete the lab in groups of 3 at Pecos and groups of 4 at Williams
PROCEDURE
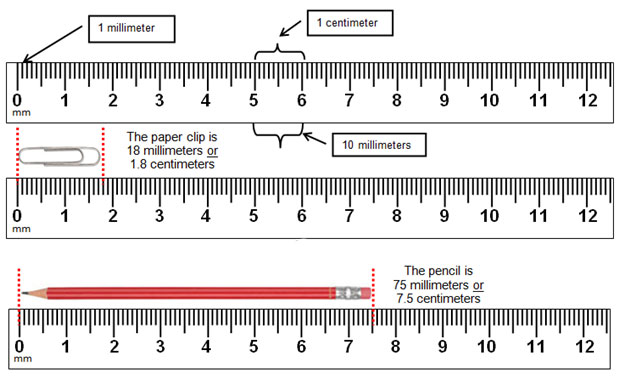
Step 1. Observe the plates. A bacterial lawn will look like a cloudy film on top of the agar. If the antiseptic or disinfectant prevented the growth of bacteria, you will see a clear zone around the disk. Measure the diameter of the zone of inhibition in millimeters (millimeters are the smallest lines on the metric side of the ruler). If there is no zone around the disk, record it as 0 mm.
Step 2. Record the results in the Antiseptics and Disinfectants worksheet.
POST TEST
DISCOVERIES IN MICROBIOLOGY
DR. JOSEPH LISTER
 In the 1800’s it was common to see the following note in a surgical report, “operation successful but the patient died”. In 1864-1866, before he developed antisepsis, 46% of British surgeon Dr. Joseph Lister’s surgical patients died of infection after surgery. Many people believed “bad air”, or miasma, was responsible for infection in surgical wounds. Hospital wards were often aired out at midday as a precaution against the spread of infection via miasma. Phenol (or carbolic acid) had been used preventing decay and neutralize the stench of dead animals and human cadavers. Frederick Calvert, Alexander McDougall, and Angus Smith used a powdered form of carbolic acid as a deodorizing agent to treat municipal sewage across the United Kingdom, most notably during London’s famous “Great Stink” of 1858. Dr. Lister read the work of Ignaz Semmelweis on hand washing and Pasteur’s work on spontaneous generation and fermentation. Dr. Lister was convinced that infection of surgical wounds was due to tiny living creatures in the air entering surgical wounds He instructed surgeons under his responsibility to wear sterilized gloves and wash their hands before and after operations with a 5% carbolic acid solution. Instruments were also washed in the same solution and assistants sprayed the solution in the operating room. One of his additional suggestions was to stop using porous natural materials in manufacturing the handles of medical instruments. After implementing his new technique (1867-1870), 15% of Dr. Lister’s patients died of infection after surgery. More fortunate than many pioneers, Dr. Lister saw the almost universal acceptance of his antisepsis techniques during his life. Dr. Lister’s work was an inspiration to others. Joseph Lawrence, a chemist living in Missouri, developed an alcohol-based formula for an antiseptic mouthwash in 1879 that he named “Listerine” in his honor.
In the 1800’s it was common to see the following note in a surgical report, “operation successful but the patient died”. In 1864-1866, before he developed antisepsis, 46% of British surgeon Dr. Joseph Lister’s surgical patients died of infection after surgery. Many people believed “bad air”, or miasma, was responsible for infection in surgical wounds. Hospital wards were often aired out at midday as a precaution against the spread of infection via miasma. Phenol (or carbolic acid) had been used preventing decay and neutralize the stench of dead animals and human cadavers. Frederick Calvert, Alexander McDougall, and Angus Smith used a powdered form of carbolic acid as a deodorizing agent to treat municipal sewage across the United Kingdom, most notably during London’s famous “Great Stink” of 1858. Dr. Lister read the work of Ignaz Semmelweis on hand washing and Pasteur’s work on spontaneous generation and fermentation. Dr. Lister was convinced that infection of surgical wounds was due to tiny living creatures in the air entering surgical wounds He instructed surgeons under his responsibility to wear sterilized gloves and wash their hands before and after operations with a 5% carbolic acid solution. Instruments were also washed in the same solution and assistants sprayed the solution in the operating room. One of his additional suggestions was to stop using porous natural materials in manufacturing the handles of medical instruments. After implementing his new technique (1867-1870), 15% of Dr. Lister’s patients died of infection after surgery. More fortunate than many pioneers, Dr. Lister saw the almost universal acceptance of his antisepsis techniques during his life. Dr. Lister’s work was an inspiration to others. Joseph Lawrence, a chemist living in Missouri, developed an alcohol-based formula for an antiseptic mouthwash in 1879 that he named “Listerine” in his honor.

