GRAM-NEGATIVE BACILLI
LEARNING OBJECTIVES
Distinguish between bacteria belonging in the Family Enterobacteriaceae from non-Enterobacteriaceae
State the purpose and principle of the oxidase test, KIA agar, tryptone agar, urea agar, nitrate reduction broth, MRVP broth, citrate agar, and motility agar
Perform and interpret the oxidase test, KIA test, indole test, urease test, nitrate test, MRVP test, citrate test, and motility test
State the significance of glucose fermentation and oxidase test in identifying gram-negative bacilli
Identify a member of the Enterobacteriaceae by results of these tests
MCCCD OFFICIAL COURSE COMPETENCIES
Describe the modes of bacterial and viral reproduction and proliferation
Utilize aseptic technique for safe handling of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cell
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
MATERIALS
Students will complete the lab in groups of 3 at Pecos and groups of 4 at Williams
Cultures: Please pick up 1 of each of the numbered (1-6) gram-negative bacilli cultures.
Klebsiella aerogenes slant culture
Klebsiella pneumoniae slant culture
Escherichia coli slant culture
Alcaligenes faecalis slant culture
Proteus mirabilis slant culture
Pseudomonas aeruginosa slant culture
Media per group:
| MEDIA | PHOTO OF MEDIA |
|---|---|
| 6 Citrate agar slants |  |
| 6 KIA agar slants |  |
| 6 Motility agar |  |
| 6 MRVP broths |  |
| 6 Nitrate broths |  |
| 6 Tryptone broths |  |
| 6 Urea agar slants |  |
Equipment:
Day One-
Inoculating loop
Inoculating wire
Bacticinerators
Day Two –
Sterile swabs – 6 per lab group
Deionized water dropper bottle
Microscope slides – 6 per lab group
Wooden sticks
Clean test tubes – 6 per lab group
Sterile transfer pipettes – 6 per lab group
Day Two Reagents:
Kovac’s Reagent
Nitrate reagent A (0.8% sulfanilic acid)
Nitrate reagent B (0.6% N, N-Dimethyl-alpha-naphthylamine)
Zinc powder
Methyl red
Voges-Proskauer reagent A (alpha-napththol)
Voges-Proskauer reagent B (40% KOH)
Oxidase strips
BIOCHEMICAL TESTS ALBUM LINK
Gram-negative bacilli comprise a vast array of bacteria, yet most clinically significant gram-negative bacilli can be divided into two groups the Enterobacteriaceae and the non-Enterobacteriaceae.
Gram‑negative bacilli in the family Enterobacteriaceae are the most frequently encountered microbes in the clinical microbiology laboratory. They are often referred to as “enterics” since since they are normally found in the intestinal tract of humans and other animals. The presence of Escherichia coli was used by public health officials for many years to indicate fecal contamination of food and water supplies. Enterobacteriaceae include many genera of bacteria such as Escherichia, Klebsiella, Salmonella, Shigella, and Proteus.
Escherichia, Klebsiella, Enterobacter and Proteus as part of the normal intestinal flora and are non‑pathogens in that environment. Of course, these bacteria can cause illness under other circumstances. For example, E. coli is a common cause of urinary tract infections and Proteus has been isolated from patients with kidney stones, gangrenous wounds, and otitis media.
The truly enteric pathogens in the Enterobacteriaceae family include Salmonella, which causes typhoid fever and mild to severe gastroenteritis (inflammation of the gastrointestinal tract) due to “food poisoning” and Shigella, which causes bacterial dysentery (bloody diarrhea with fever) due to “food poisoning”.
The Enterobacteriaceae have four common characteristics:
- They are all gram-negative bacilli
- They all ferment glucose
- They do not produce oxidase
- They all reduce nitrate to nitrite
The gram-negative bacilli that are not in the Enterobacteriaceae family are composed of several families but are collectively called non-Enterobacteriaceae or non-fermenters (of glucose) to differentiate them from the Enterobacteriaceae. Non-Enterobacteriaceae include bacteria such as Pseudomonas aeruginosa and Alcaligenes faecalis. These bacteria are mostly found in the environment, yet some of these organisms can cause wound infections and serious life-threatening infections in immunocompromised patients.
The non-Enterobacteriaceae also have three common characteristics:
- They are all gram-negative
- They do not ferment glucose
- Many (but not all) are oxidase positive
To identify the gram-negative bacilli, we will perform biochemical tests to detect the presence or absence of metabolic enzymes. Substrates upon which these enzymes act are incorporated into the culture medium along with an indicator system to allow detection of specific metabolic products.
PRE-ASSESSMENT
PROCEDURE
For this exercise: Work in groups of 3 at Pecos groups of 4 at Williams. Perform the following tests on each color dot culture.
NITRATE Broth REDUCTION TEST
Nitrate broth is used to determine if an organism can reduce nitrate. Some bacteria can reduce nitrate (NO3) to nitrite (NO2) by producing the enzyme nitrate reductase. Other bacteria can reduce nitrate to nitrogen gas by also producing the enzyme nitrite reductase which reduces nitrite to nitrogen gas. Other bacteria do not have the ability to reduce nitrate at all.
Nitrate —-nitrate reductase—-> Nitrite —-nitrite reductase—-> Nitrogen Gas
Step 1. Obtain a nitrate broth. Use a permanent marker to write the unknown number, your name (or initials), and the name of the media (nitrate) on the side of the tube.
Step 2. Using a sterile inoculating loop, obtain a small amount of bacteria and inoculate the nitrate broth.
Step 3. Incubate the nitrate broth in the class test tube rack.
AFTER INCUBATION –Add reagents to the incubated nitrate broth. Add 10 drops of nitrate reagent A (0.8% sulfanilic acid) and then add 10 drops of nitrate reagent B (0.6% N, N-Dimethyl-alpha-naphthylamine). IMMEDIATELY look for a dark red color which indicates the bacteria used the nitrate reductase enzyme to reduce nitrate to nitrite. This is a positive test result, and no further testing is required. Record your reaction and results on the worksheet. The nitrate reagents have an unpleasant odor. Dispose of the nitrate broth in the labeled rack in the fume hood.
If no red color occurs – Dip a wooden applicator stick in zinc powder (just enough to get the stick “dirty”) and drop the wooden applicator stick into the nitrate broth. The zinc powder reduces nitrate to nitrite. A red color after the addition of zinc is a negative test result. Nitrate was not reduced by the bacteria, it was reduced by zinc. If a red color does not occur after the addition of zinc, there was no nitrate for zinc to reduce. This is considered a positive result since the bacteria reduced nitrate to nitrogen gas. Record your reaction and results on the worksheet. The nitrate reagents have an unpleasant odor. Dispose of the nitrate broth in the labeled rack in the fume hood.

After adding Nitrate Reagent A and Nitrate Reagent B
Red color = Positive (Nitrate was reduced to Nitrite)
If no color, then add zinc
No color = Positive (Nitrate was reduced to Nitrogen gas)
Red color = Negative (Nitrate was not reduced by the bacteria)

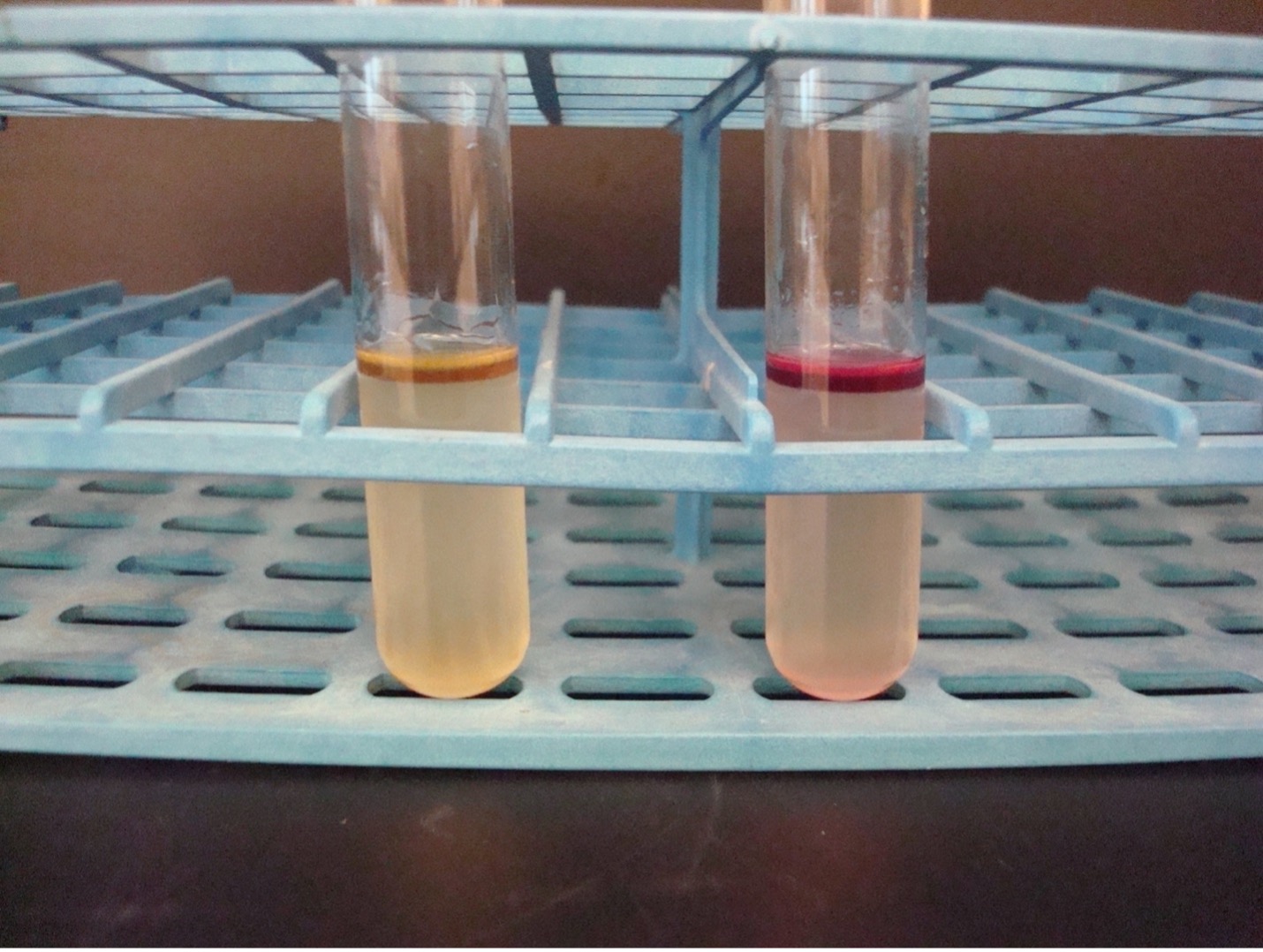
UREASE TEST
This test detects the ability of the bacteria to produce the enzyme urease. The media contains urea and the pH indicator phenol red. Urease will hydrolyze urea to produce ammonia. The alkaline environment will change the phenol red indicator to hot pink.
Step 1. Obtain a urea agar slant. Use a permanent marker to write the unknown number, your name (or initials), and the name of the media (urea) on the side of the tube.
Step 2. Using a sterile inoculating loop, obtain a heavy amount of bacteria and streak the entire surface of the urea agar.
Step 3. Incubate the inoculated slant in the class test tube rack until next lab session.
AFTER INCUBATION-Observe the urea media for a color change. Record the result on the worksheet. Dispose of the urea slant in the discard rack.
Hot pink media= Positive
Salmon or Light Pink = Negative
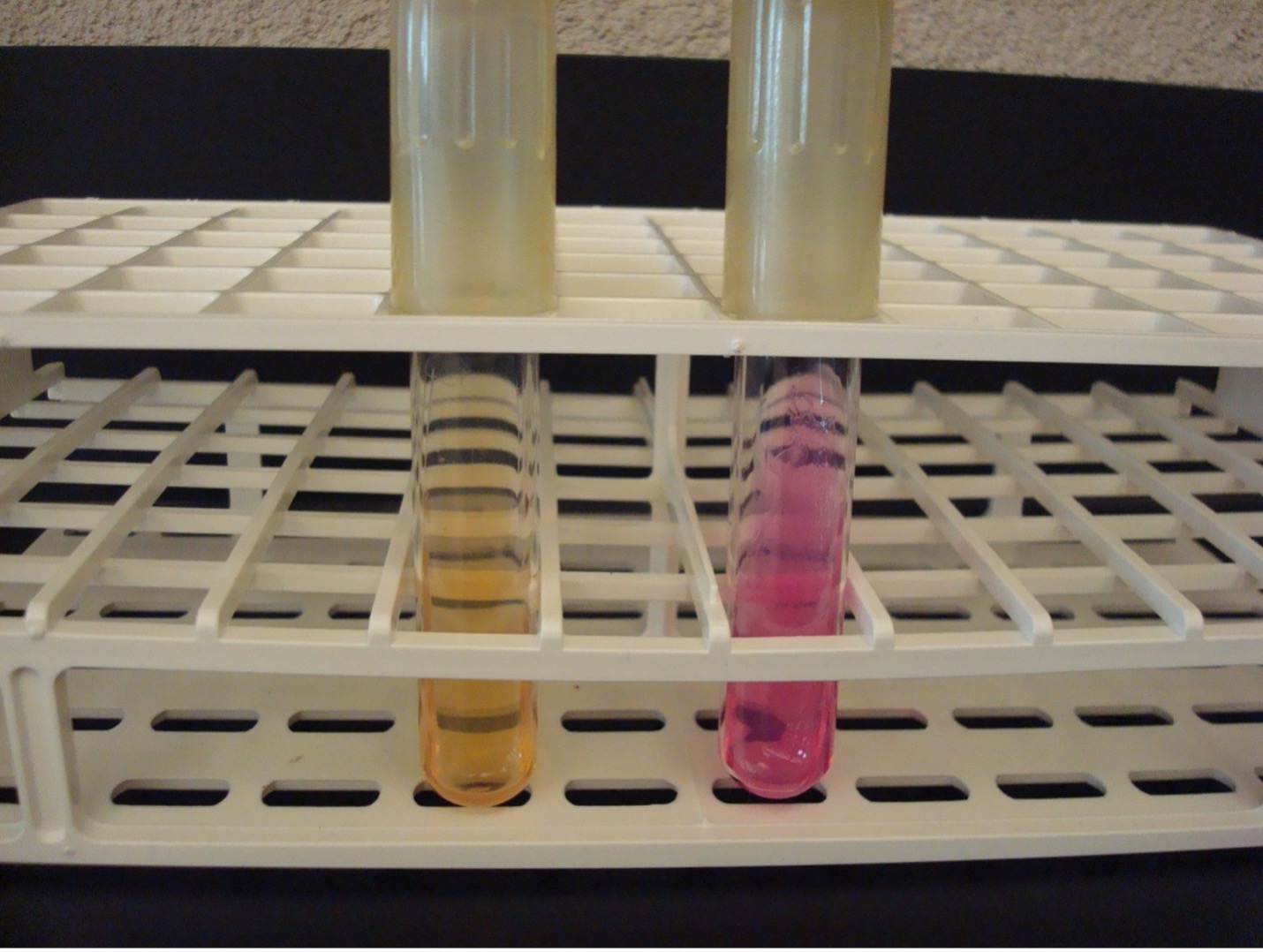
INDOLE TEST using tryptone broth
The Indole test uses tryptone broth to detect the bacterias’s ability to produce tryptophanase. This enzyme will split the amino acid tryptophan into indole and pyruvic acid. Indole forms a red ring with the addition of Kovac’s reagent.
Step 1. Obtain a tryptone broth. Use a permanent marker to write the unknown number, your name (or initials), and the name of the media (tryptone) on the side of the tube.
Step 2. Using a sterile inoculating loop, obtain a small amount of bacteria and inoculate the culture into the tryptone broth.
Step 3. Incubate the inoculated broth in the class test tube rack until next lab session.
AFTER INCUBATION-Add 10 drops of Kovac’s reagent to the tryptone tube. Record the reaction/results on the worksheet. Kovac’s reagent has an unpleasant odor. Dispose of the tryptone broth in the in the labeled rack in the fume hood. .
Red ring = Positive
No red ring = Negative
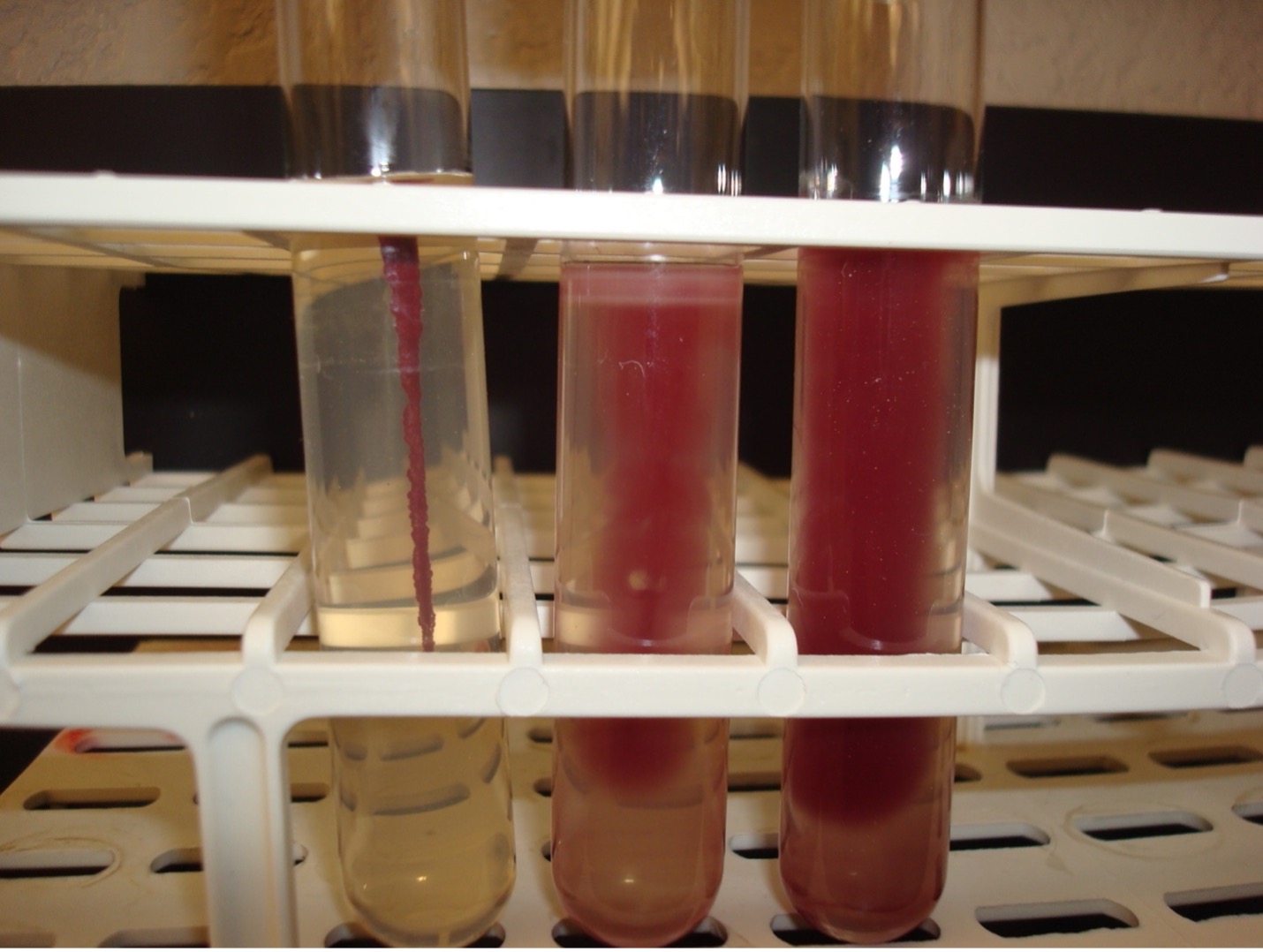
MOTILITY TEST
The motility test detects the ability of bacteria to produce flagella. The media is a semi-solid agar with a colorless dye. As bacteria grow, the dye is reduced by the bacteria and turns red. Bacterial growth is detected by the presence of the red color.
Step 1. Obtain a motility agar. Use a permanent marker to write the unknown number, your name (or initials), and the name of the media (motility) on the side of the tube.
Step 2. Using a sterile inoculating WIRE, obtain a small amount of bacteria. Carefully stab the media about two thirds of the way down. Keep the wire in the same line it entered as you remove it from the media.
Step 3. Incubate the inoculated agar in the class test tube rack until next lab session.
AFTER INCUBATION-Observe the bacterial growth in the media. If the bacteria migrate away from the inoculation stab, the red dye will diffuse in the tube where the bacteria are growing. If the bacteria are not able to migrate, the red dye will be seen only in the stab line where the bacteria are growing. Record the reaction and results on the worksheet. Dispose of the motility agar in the discard rack.
Diffused red color = Positive
Red color only at the stab line = Negative
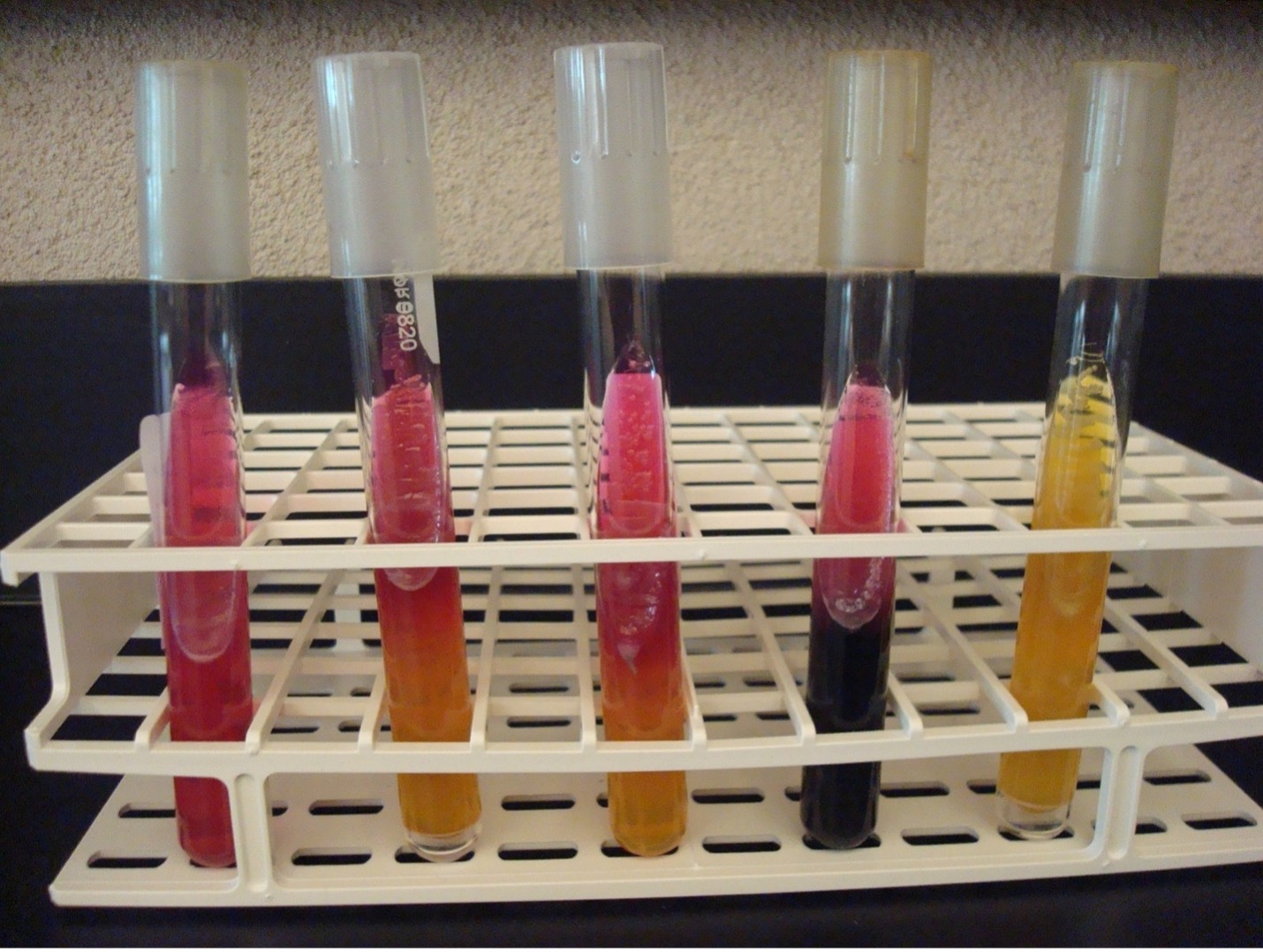
METHYL RED–VOGES PROSKAUER (MRVP) broth TEST
The MRVP test is two separate tests that uses one broth of glucose to determine the end product of glucose fermentation. Some bacteria ferment glucose using mixed acid fermentation pathway (detected in the MR test) while other bacteria ferment glucose via the butanediol fermentation pathway (detected in the VP test).
MR-VP Broth (Glucose) —–> Pyruvic acid ——> Mixed acid fermentation pathway (MR+)
OR
MR-VP Broth (Glucose) —–> Pyruvic acid ——> Butanediol fermentation pathway (VP+)
Step 1. Obtain a MRVP broth tube. Use a permanent marker to write the unknown number, your name (or initials), and the name of the media (MRVP) on the side of the tube.
Step 2. Using a sterile inoculating loop, obtain a small amount of bacteria and inoculate the MRVP broth.
Step 3. Incubate the inoculated broth in the class test tube rack until next lab session.
AFTER INCUBATION
Step 1. Use a sterile transfer pipette to transfer half of the inoculated MRVP broth to a clean test tube. Label this tube “VP”. Leave the other half of the inoculated broth in the original MRVP broth test tube. Label this tube “MR”.
Step 2. MR TEST Add 10 drops of methyl red reagent to “MR” tube. Methyl red is a pH indicator that will indicate the bacteria fermented glucose via the mixed acid fermentation pathway. Record the reactions and results on the worksheet. Methyl red has an unpleasant odor. Dispose of the MR broth in the in the labeled rack in the fume hood.
Step 3. VP TEST First add 15 drops of Voges-Proskauer reagent A (alpha-napththol) and then add 5 drops of Voges-Proskauer reagent B (40% KOH) to the “VP” tube. NOTE: A reversal in the order of the reagents may result in a weak-positive or false-negative reaction. Mix the tube well by flicking it with your fingers and then let it sit undisturbed in a test tube rack for 20 minutes. A positive reaction indicates the bacteria ferment glucose via the butanediol fermentation pathway. Record the reactions and results on the worksheet. Voges-Proskauer reagents A and B have an unpleasant odor. Dispose of the VP broth in the in the labeled rack in the fume hood.
Red broth = MR Positive
Yellow broth = MR Negative

Dark red-brown color at the top of the tube = VP Positive
Yellow color at the top of the tube = VP Negative

CITRATE TEST
This test uses citrate agar to determine if the bacteria can use citrate as its only carbon source. Since all organisms need carbon to survive, bacteria that cannot use the citrate will not grow. The agar contains citrate and ammonium ions (nitrogen source) and bromthymol blue as a pH indicator.
Step 1. Obtain a citrate agar slant. Use a permanent marker to write the unknown number, your name (or initials), and the name of the media (citrate) on the side of the tube.
Step 2. Using a sterile inoculating loop, obtain a large amount of bacteria and streak the entire slant of the slant.
Step 3. Incubate the inoculated slant in the class test tube rack until next lab session.
AFTER INCUBATION-Observe the color of the citrate slant. Record the reaction and results on the worksheet. Dispose of the citrate slant in the discard rack.
Bright blue = Positive
No color change (stays green) = Negative

KLIGLER IRON AGAR (KIA)
KIA media contains nutrient agar, ferrous sulfate, phenol red pH indicator, glucose, and lactose. Lactose is present in ten times the concentration of glucose. KIA media is used to determine if bacteria can ferment glucose and lactose with or without gas production. The media can also determine if bacteria can produce hydrogen sulfide. If bacteria cannot ferment glucose, they are called “non-fermenters”. If bacteria can ferment glucose, they are called “fermenters”. If bacteria can ferment glucose, then it is possible that it can also ferment lactose or produce hydrogen sulfide.
Step 1. Obtain a KIA tube and a straight inoculating wire. Use a permanent marker to write the unknown number, your name (or initials), and the name of the media (KIA) on the side of the tube.
Step 2. Use the sterile straight inoculating wire to take an inoculum of the bacteria.
Step 3. Begin by stabbing in the middle of the KIA slant to just above (3-5mm or 0.25 inch) the bottom of the tube. As the needle is withdrawn from the agar, streak the entire surface of the agar slant.
Step 4. Incubate the KIA slant in the class test tube rack.
AFTER INCUBATION –Observe the reactions in the KIA tube and record the reactions/results. Record the reactions and results on the worksheet. Use the growth on the KIA slant to perform the oxidase test.
Butt of KIA tube:
Yellow butt = Positive glucose fermentation (a black precipitate may mask the yellow color)
Red butt = Negative glucose fermentation
Gas production from glucose fermentation:
Bubbles or splitting of the agar = Positive for gas (if bacteria cannot ferment glucose, they will not produce gas)
Slant of the KIA tube:
Yellow slant = Positive lactose fermentation
Red slant = Negative lactose fermentation
Hydrogen sulfide production:
Black precipitate in the media = Positive Hydrogen Sulfide Production
OXIDASE TEST (use the growth on THE kia agar slant for this lab exercise or your gram-negative stock for the mixed unknown)
This test determines if the bacteria can produce the enzyme cytochrome oxidase which transfers electrons in the electron transport chain in cellular respiration. The bacteria to be tested must be grown on non-inhibitory media such as KIA or TSA because the production of oxidase is easily inhibited by selective media.
Step 1. Place the oxidase strip face up (look at the edges of the strip, they should be pointing down) on a glass slide. Barely moisten the oxidase strip with a small drop of water. Do not over-moisten the strip!
Step 2. Using a sterile swab, obtain a large amount of inoculum from the KIA slant (this lab exercise) or TSA plate (the mixed unknown lab exercise). Rub the bacteria on the swab on the oxidase strip.
Step 3. Watch for purple color to develop on the strip or swab within 20 seconds. Record the reaction/result on the worksheet. Dispose of the oxidase strip and swab in the biohazard trash. Dispose of the slide in the used slide basin. Dispose of the KIA slant is the discard rack.
Purple color change = Positive
No color change within 20 seconds = Negative

GRAM NEGATIVE-BACILLI BIOCHEMICAL TEST RESULTS
| TEST | Escherichia coli | Klebsiella aerogenes |
Klebsiella pneumoniae |
Proteus mirabilis | Pseudomonas aeruginosa | Citrobacter freundii |
|---|---|---|---|---|---|---|
| Oxidase | Negative | Negative | Negative | Negative | Positive | Negative |
| Glucose fermentation | Positive | Positive | Positive | Positive | Negative | Positive |
| Lactose fermentation | Positive | Negative | Negative | Negative | Negative | Positive |
| H2S production | Negative | Negative | Negative | Positive | Negative | Negative |
| Nitrate reduction | Positive | Positive | Positive | Positive | Positive | Positive |
| Urease | Not reliable | Positive | Positive | Positive | Not reliable | Not reliable |
| Indole | Positive | Negative | Negative | Negative | Not reliable | Negative |
| Motility | Positive | Positive | Negative | Positive | Not reliable | Positive |
| MR Test | Positive | Negative | Negative | Positive | Negative | Positive |
| VP Test | Negative | Not reliable | Positive | Negative | Negative | Negative |
| Citrate | Negative | Positive | Positive | Positive | Positive | Positive |
POST TEST
DISCOVERIES IN MICROBIOLOGY
DR. BARUJ BENACERRAF

In the 1940’s, Venezuelan immunologist Dr. Benacerraf discovered the major histocompatibility complex genes which encode cell surface protein molecules critical to the immune system’s distinction between self and non-self. Benacerraf also helped develop our understanding of the phagocytic activity of macrophages, described the functions of IgG subclasses, discovered Fc receptors, demonstrated that T and B cells recognize antigens differently, and observed how B and T cells cooperate with each other to generate an antibody response.

