14 Energy Sources and Air Pollution
Learning Objectives
After studying this chapter, you should be able to:
- Outline the history of human energy use
- Understand the challenges to continued reliance on fossil energy
- Outline environmental impacts of energy use
- Evaluate the different energy sources based on their environmental impact
- List common air pollutants and their sources
- Explain the impact of human activity on the ozone layer
- Describe how acid rain forms and causes environmental damage
Energy Sources
Energy for lighting, heating and cooling our buildings, manufacturing products, and powering our transportation systems comes from a variety of natural sources. The earth’s core provides geothermal energy. The gravitational pull of moon and sun create tides. The sun emits light (electromagnetic radiation), which creates wind, powers the water (hydrologic) cycle, and enables photosynthesis. Plants, algae, and cyanobacteria utilize solar energy to grow and create biomass that can be burned and used for biofuels, such as wood, biodiesel, bioethanol. Over the course of millions of years, biomass from photosynthetic organisms can create energy-rich fossil fuels through the geologic process of burial and transformation through heat and pressure.
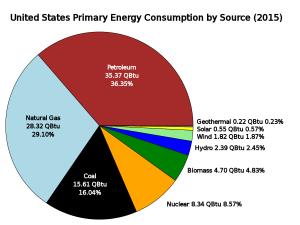
Each of these types of energy can be defined as renewable or non-renewable. Renewable energy sources can be replenished within human lifespans. Examples include solar, wind, and biomass energy. Non-renewable energy is finite and cannot be replenished within a human timescale. Examples include nuclear energy and fossil fuels, which take millions of years to form. All energy sources have and some environmental and health cost, and the distribution of energy is not equally distributed among all nations. The United States generates less than 10% of its energy from renewable sources (Figure 1), while Costa Rica generates more than 99% of its electricity from renewables.
Non-Renewable Energy Sources
Fossil fuels come from the organic matter of plants, algae, and cyanobacteria that was buried, heated, and compressed under high pressure over millions of years. The process transformed the biomass of those organisms into the three types of fossil fuels: oil, coal, and natural gas.
Petroleum (oil)
Thirty seven percent of the world’s energy consumption and 36.35% of the United States energy consumption comes from oil. Scientists and policy-makers often discuss the question of when the world will reach peak oil production, the point at which oil production is at its greatest and then declines. It is generally thought that peak oil will be reached by the middle of the 21st Century, although making such estimates is difficult because a lot of variables must be considered. Currently world reserves are 1.3 trillion barrels, or 45 years left at current level of production.
Oil is usually found one to two miles (1.6 – 3.2 km) below the Earth’s surface, whether that is on land or ocean. Once oil is found and extracted it must be refined, which separates and prepares the mix of crude oil into the different types for gas, diesel, tar, and asphalt. Oil refining is one of top sources of air pollution in the United States for volatile organic hydrocarbons and toxic emissions, and the single largest source of carcinogenic benzene. When petroleum is burned as gasoline or diesel, or to make electricity or to power boilers for heat, it produces a number of emissions that have a detrimental effect on the environment and human health, discussed in detail later in this chapter.
There are other domestic sources of oil that are being considered as conventional resources and are being depleted. These include tar sands – deposits of moist sand and clay with 1-2 percent bitumen (thick and heavy petroleum rich in carbon and poor in hydrogen). These are removed by strip mining (see section below on coal). Another source is oil shale, which is sedimentary rock filled with organic matter that can be processed to produce liquid petroleum. Extracted by strip mining or creating subsurface mines, oil shale can be burned directly like coal or baked in the presence of hydrogen to extract liquid petroleum. However, the net energy values are low and they are expensive to extract and process. Both of these resources have severe environmental impacts due to strip mining, carbon dioxide, methane and other air pollutants similar to other fossil fuels.
As the United States tries to extract more oil from its own dwindling resources, oil companies are drilling even deeper into the earth and increasing the environmental risks. The largest United States oil spill to date began in April 2010 when an explosion occurred on Deepwater Horizon Oil Rig in the Gulf of Mexico killing 11 employees and spilling nearly 200 million gallons of oil before the resulting leak could be stopped (Figure 2). Wildlife, ecosystems, and people’s livelihood were adversely affected. Millions of dollars and huge amounts of energy were expended on immediate clean-up efforts. The long-term impacts are still not known.

Coal
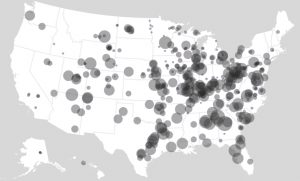
Coal is another fossil fuel, but unlike oil it is a solid. Due to its relatively low cost and abundance, coal is used to generate about 15.6% of the electricity consumed in the United States. Coal production doubled in the United States between 1950 and 2010 and is burned as an energy source in almost every state (Figure 3). However, the use of coal has decreased in recent years as natural gas and renewable sources have become cheaper and more widespread. Current world reserves are estimated at 826,000 million metric tons (182 million pounds), with nearly 30% of that in the United States. It is a major fuel resource that the United States controls domestically.
Coal is plentiful and inexpensive, when looking only at the market cost relative to the cost of other sources of electricity, but its extraction, transportation, and use produces a multitude of negative externalities that the market cost does not truly capture. Coal emits sulfur dioxide, nitrogen oxide, and mercury, which have been linked to acid rain, smog, and health issues. Burning of coal emits higher amounts of carbon dioxide per unit of energy than the use of oil or natural gas. Coal accounted for 35% of the total United States emissions of carbon dioxide released into the Earth’s atmosphere in 2010. Ash generated from combustion contributes to water contamination. Some coal mining has a negative impact on ecosystems and water quality, and alters landscapes and scenic views (such as with mountaintop mining).
There are also significant health effects and risks to coal miners and those living in the vicinity of coal mines. Traditional underground mining is risky to mine workers due to the risk of entrapment or death. Over the last 15 years, the U.S. Mine Safety and Health Administration has published the number of mine worker fatalities and it has varied from 18-48 per year. Twenty-nine miners died on April 6, 2010 in an explosion at the Upper Big Branch coal mine in West Virginia, contributing to the uptick in deaths between 2009 and 2010. In other countries, with less safety regulations, accidents occur more frequently. In May 2011, for example, three people died and 11 were trapped in a coalmine in Mexico for several days. There is also risk of getting black lung disease (pneumoconiosis). This is a disease of the lungs caused by the inhalation of coal dust over a long period of time. It causes coughing and shortness of breath and is responsible for tens of thousands of deaths per year.
Mountaintop mining (MTM), while less hazardous to workers, has particularly detrimental effects on land resources. MTM is a surface mining practice involving the removal of mountaintops to expose coal seams, and disposing of the associated mining waste in adjacent valleys. This form of mining is very damaging to the environment because it literally removes the tops of mountains, destroying the existing habitat. Additionally, the debris from MTM is dumped into valleys, burying streams and other important habitat (Figure 4).

Natural Gas
Natural gas meets 20% of world energy needs and 28% of United States needs. Natural gas is mainly composed of methane (CH4) and is a very potent greenhouse gas that contributes to global warming. Natural gas is released into the atmosphere from coal mines, oil and gas wells, and natural gas storage tanks, pipelines, and processing plants. These leaks are the source of about 25% of total U.S. methane emissions, which translates to three percent of total U.S. greenhouse gas emissions. When natural gas is produced but cannot be captured and transported economically, it is “flared,” or burned at well sites, which converts it to CO2. This is considered to be safer and better than releasing methane into the atmosphere because CO2 is a less potent greenhouse gas than methane.
Natural gas is often considered a preferred fossil fuel compared to oil or coal when considering its environmental impacts. Specifically, when burned, much less carbon dioxide (CO2), nitrogen oxides, and sulfur dioxide are emitted than from the combustion of coal or oil. It also does not produce ash or toxic emissions. However, there is still a great deal of environmental damage associated with natural gas production. Most natural gas in the United States is now extracted from deep layers of rocks known as shale. Extraction of shale gas is more problematic than traditional sources due to a process known as hydraulic fracturing, or fracking (Figure 5). The technique uses high-pressure fluids to fracture the normally hard shale deposits and release gas and oil trapped inside the rock. To promote the flow of gas out of the rock, small particles of solids are included in the fracturing liquids to lodge in the shale cracks and keep them open after the liquids are depressurized. The considerable use of water may affect the availability of water for other uses in some regions and this can affect aquatic habitats. If mismanaged, hydraulic fracturing fluid can be released by spills, leaks, or various other exposure pathways. The fluid contains potentially hazardous chemicals such as hydrochloric acid, glutaraldehyde, petroleum distillate, and ethylene glycol.
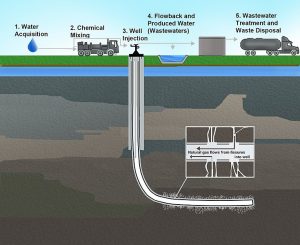
The raw gas from a well may contain many other compounds besides the methane that is being sought, including hydrogen sulfide, a very toxic gas. Natural gas with high concentrations of hydrogen sulfide is usually flared which produces CO2, carbon monoxide, sulfur dioxide, nitrogen oxides, and many other compounds. Natural gas wells and pipelines often have engines to run equipment and compressors, which produce additional air pollutants and noise.
Nuclear Power
Nuclear power is energy released from the radioactive decay of elements, such as uranium, which releases large amounts of energy. Currently, world production of electricity from nuclear power is about 19.1 trillion KWh, with the United States producing and consuming about 22% of that. Nuclear power provides just over 8% of the total electricity used in this country.
Although nuclear power generation does not release greenhouse gases, there are environmental challenges with nuclear power. Mining and refining uranium ore and making reactor fuel demands a lot of energy. Also, nuclear power plants are very expensive and require large amounts of metal, concrete, and energy to build. The main environmental challenge for nuclear power is the leftover waste including uranium mine waste, spent (used) reactor fuel, and other radioactive wastes. These materials persist in the environment for thousands of years and thus will remain a threat to human health long into the future.
By volume, the waste produced from mining uranium, called uranium mill tailings, is the largest source of nuclear waste and contains the radioactive element radium, which decays to produce radon, a radioactive gas. High-level radioactive waste consists of used nuclear reactor fuel. This fuel is in a solid form consisting of small fuel pellets in long metal tubes and must be stored and handled with multiple containment, first cooled by water and later in special outdoor concrete or steel containers that are cooled by air. There is no long-term storage facility for this fuel in the United States.
There are many other regulatory precautions governing permitting, construction, operation, and decommissioning of nuclear power plants due to risks from an uncontrolled nuclear reaction. The potential for contamination of air, water and food is high should an uncontrolled reaction occur. Even when planning for worst-case scenarios, there are always risks of unexpected events. For example, the March 2011 earthquake and subsequent tsunami that hit Japan resulted in reactor meltdowns at the Fukushima Daiichi Nuclear Power Station, causing massive damage to the surrounding area (Figure 6).

Renewable Energy Sources
Hydropower
Hydropower (hydroelectric) relies on water flowing through a dam to spin turbines and create electricity (Figure 7). It is considered a clean and renewable source of energy because it does not directly produce pollutants and because the source of power is regenerated. Hydropower provides about 25% of the United States’ renewable energy consumption.

Hydropower dams and the reservoirs they create can have environmental impacts. For example, migration of fish to their upstream spawning areas can be obstructed by dams. In areas where salmon must travel upstream to spawn, such as along the Columbia River in Washington and Oregon, the dams block their way. This problem can be partially alleviated by using “fish ladders” that help salmon get around the dams. Fish traveling downstream, however, can get killed or injured as water moves through turbines in the dam. Reservoirs and operation of dams can also affect aquatic habitats due to changes in water temperatures, water depth, chemistry, flow characteristics, and sediment loads, all of which can lead to significant changes in the ecology and physical characteristics of the river both upstream and downstream. As reservoirs fill with water it may cause natural areas, farms, cities, and archeological sites to be inundated and force populations to relocate.
Liquid Biofuels
Bioethanol and biodiesel are two types of lIquid biofuels manufactured from plants to replace fossil fuels. Bioethanol can be easily fermented from sugar cane juice, as is done in Brazil, and can also be fermented from broken down corn starch, as is mainly done in the United States. The economic and social effects of growing plants for fuels need to be considered, since the land, fertilizers, and energy used to grow biofuel crops could be used to grow food crops instead. The competition of land for fuel vs. food can increase the price of food, which has a negative effect on society. It could also decrease the food supply increasing malnutrition and starvation globally. Also, in some parts of the world, large areas of natural vegetation and forests have been cut down to grow sugar cane for bioethanol and soybeans and palm-oil trees to make biodiesel. This is not sustainable land use. Biofuels may be derived from parts of plants not used for food, such as stalks, thus reducing that impact. Biodiesel can be made from used vegetable oil and has been produced on a very local basis. Compared to petroleum diesel, biodiesel combustion produces less sulfur oxides, particulate matter, carbon monoxide, and unburned and other hydrocarbons, but it produces more nitrogen oxide.
Geothermal Energy
Five percent of the United States’ renewable energy comes from geothermal energy: using the heat of Earth’s subsurface to provide endless energy. Geothermal systems utilize a heat-exchange system that runs in the subsurface about 20 feet (5 meters) below the surface where the ground is at a constant temperature. The system uses the earth as a heat source (in the winter) or a heat sink (in the summer). This reduces the energy consumption required to generate heat from gas, steam, hot water, and conventional electric air-conditioning systems. The environmental impact of geothermal energy depends on how it is being used. Direct use and heating applications have almost no negative impact on the environment.
Geothermal power plants do not burn fuel to generate electricity so their emission levels are very low. They release less than 1% of the carbon dioxide emissions of a fossil fuel plant. Geothermal plants use scrubber systems to clean the air of hydrogen sulfide that is naturally found in the steam and hot water. They emit 97% less acid rain-causing sulfur compounds than are emitted by fossil fuel plants. After the steam and water from a geothermal reservoir have been used, they are injected back into the earth. Although geothermal tends to be a very sustainable source of energy, it is only available in limited areas that have specific underground geological formations.
Solar Energy
Solar power converts the energy of light into electrical energy and has minimal impact on the environment, depending on where it is placed. In 2015, 5.6% of the renewable energy generated in the United States was from solar power out of the 9.68% of the total electricity generation that was from renewable sources. Solar power generation releases no carbon emissions or other air pollutants, but the manufacturing of photovoltaic (PV) cells generates some hazardous waste from the chemicals and solvents used in processing. Often solar arrays are placed on roofs of buildings or over parking lots or integrated into construction in other ways (Figure 8). However, large systems may be placed on land and particularly in deserts where those fragile ecosystems could be damaged if care is not taken. Some solar thermal systems use potentially hazardous fluids (to transfer heat) that require proper handling and disposal. Concentrated solar systems may need to be cleaned regularly with water, which is also needed for cooling the turbine generator. Using water from underground wells may affect the ecosystem in some arid locations.

Wind Energy
Wind energy is a renewable energy source that is clean and has very few environmental challenges. Wind turbines are becoming a more prominent sight across the United States, even in regions that are considered to have less wind potential. Wind turbines (often called windmills) do not release emissions that pollute the air or water (with rare exceptions), and they do not require water for cooling. The U.S. wind industry had 40,181 MW of wind power capacity installed at the end of 2010, with 5,116 MW installed in 2010 alone, providing more than 20% of installed wind power around the globe. According to the American Wind Energy Association, over 35% of all new electrical generating capacity in the United States since 2006 was due to wind, surpassed only by natural gas (Figure 9).

Because a wind turbine has a small physical footprint relative to the amount of electricity it produces, many wind farms are located on crop and pasture land. They contribute to economic sustainability by providing extra income to farmers and ranchers, allowing them to stay in business and keep their property from being developed for other uses. For example, energy can be produced by installing wind turbines in the Appalachian mountains of the United States instead of engaging in mountain top removal for coal mining. Offshore wind turbines on lakes or the ocean may have smaller environmental impacts than turbines on land.
Wind turbines do have a few environmental challenges. There are aesthetic concerns to some people when they see them on the landscape. A few wind turbines have caught on fire, and some have leaked lubricating fluids, though this is relatively rare. Some people do not like the sound that wind turbine blades make. Turbines have been found to cause bird and bat deaths, particularly if they are located along their migratory path. This is of particular concern if these are threatened or endangered species. Ways to mitigate that impact are currently being researched. There are some small impacts from the construction of wind projects or farms, such as the construction of service roads, the production of the turbines themselves, and the concrete for the foundations. However, overall analysis has found that turbines make much more energy than the amount used to make and install them.
Knowledge Check
Air Pollution
Air pollution occurs in many forms but can generally be thought of as gaseous and particulate contaminants that are present in the earth’s atmosphere. Chemicals discharged into the air that have a direct impact on the environment are called primary pollutants. These primary pollutants sometimes react with other chemicals in the air to produce secondary pollutants.
Five of the most common primary air pollutants are carbon monoxide, sulfur dioxide, nitrogen oxides, volatile organic compounds, and particulate matter. Ground-level ozone is a secondary pollutant. These pollutants can harm health and the environment, and cause property damage. Of the six pollutants, particle pollution and ground-level ozone are the most widespread health threats. The U.S. Environmental Protection Agency (EPA) regulates them by developing criteria based on considerations of human and environmental health.
Carbon monoxide (CO) is a colorless, odorless gas emitted from combustion processes. Nationally, particularly in urban areas, the majority of CO emissions to ambient air come from automobiles. CO can cause harmful health effects by reducing oxygen delivery to the body’s organs (like the heart and brain) and tissues. At extremely high levels, CO can cause death.
Sulfur dioxide (SO2) is a highly reactive gas that is the main cause of acid rain (discussed below). The largest sources of SO2 emissions are from fossil fuel combustion at power plants (73%) and other industrial facilities (20%). Smaller sources of SO2 emissions include industrial processes such as extracting metal from ore, and the burning of high sulfur containing fuels by locomotives, large ships, and non-road equipment. SO2 is linked with a number of adverse effects on the respiratory system.
Nitrogen oxides (NOx) are another group of highly reactive gases that includes various combinations of nitrogen and oxygen, including nitrogen dioxide (NO2) and nitrous oxide (N2O). Nitrogen oxides form quickly from emissions from cars, trucks and buses, power plants, and off-road equipment. In addition to contributing to the formation of ground-level ozone, nitrogen oxides are linked with a number of adverse effects on the respiratory system.
Volatile organic compounds (VOCs) are a group of thousands of carbon-containing compounds that may be natural or created by humans. Major human sources of VOCs are burning of fossil fuels, industrial processes, and paints. VOCs are responsible for most scents, from “new car smell” to the pleasant aroma of roses. Most of these compounds are harmless to humans, but high concentrations, especially inside buildings, can lead to health problems such as cancer.
Particulate matter is a complex mixture of extremely small particles and liquid droplets. Particulate matter pollution is made up of a number of components, including acids (such as nitrates and sulfates), organic chemicals, metals, and soil or dust particles. Particulate matter is responsible for smog, or pollution that reduces visibility. The size of particles is directly linked to their potential for causing health problems. The EPA classifies the most harmful particulate matter into two size classes, PM2.5 and PM10. PM2.5 refers to particles that are less than 2.5 micrometers in diameter, while PM10 refers to particles between 2.5 and 10 micrometers. For reference, a human hair is about 70 micrometers in diameter. The smaller the particle, the more easily it can penetrate the lungs. Once inhaled, these particles can affect the heart and lungs and cause serious health effects.
Ground-level ozone (O3) is considered a secondary pollutant because it is not emitted directly into the air, but is created by chemical reactions between nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. Breathing ozone can trigger a variety of health problems, particularly for children, the elderly, and people of all ages who have lung diseases such as asthma. Ground-level ozone can also have harmful effects on sensitive vegetation and ecosystems. Ground-level ozone should not be confused with the ozone layer, which is high in the atmosphere and protects Earth from ultraviolet light (discussed in the next section).
Knowledge Check
Ozone Depletion
Unlike ground-level ozone, which is harmful, the ozone layer is a band of ozone high in the atmosphere that protects humans, plants, and wildlife from damaging ultraviolet (UV) radiation emitted from the sun (Figure 10).
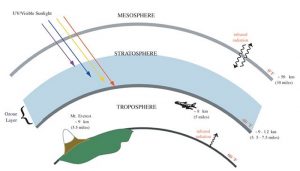
Ozone is constantly produced and destroyed in a natural cycle powered by the sun. Normally, levels of ozone remain constant over time, but human activity has upset the balance through the production of ozone-depleting substances, such as chlorofluorocarbons (CFCs). These compounds were commonly used as refrigerants, degreasing solvents, and propellants in aerosol cans beginning in the 1920s. CFCs are extremely stable, and when they are released they float up into the stratosphere, reaching the ozone layer within a few years. At that point, the CFCs react with ozone and turn it into normal oxygen (O2), which is incapable of blocking the sun’s UV rays. This allows more UV radiation to reach the earth’s surface, leading to higher levels of skin cancer in humans and damage to the cells of other animals and plants.
One success story in reducing pollutants that harm the atmosphere concerns ozone-destroying substances. In 1973, scientists calculated that CFCs could reach the stratosphere and break apart. This would release chlorine atoms, which would then destroy ozone. Based only on their calculations, the United States and most Scandinavian countries banned CFCs in spray cans in 1978. More confirmation that CFCs break down ozone was needed before more was done to reduce production of ozone-destroying chemicals. In 1985, members of the British Antarctic Survey reported that a 50% reduction in the ozone layer had been found over Antarctica, an area now known as the ozone hole (Figure 11).
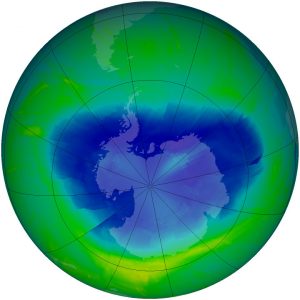
Two years after the British Antarctic Survey discovered the ozone hole, the Montreal Protocol on Substances that Deplete the Ozone Layer was ratified by nations all over the world. The Montreal Protocol controls the production and consumption of 96 chemicals that damage the ozone layer. CFCs have been mostly phased out since 1995, although they were used in developing nations until 2010. Some of the less hazardous substances will not be phased out until 2030. The Protocol also requires that wealthier nations donate money to develop technologies that will replace these chemicals.
Because CFCs take many years to reach the stratosphere and can survive there a long time before they break down, the ozone hole will probably continue to grow for some time before it begins to shrink. The stratosphere is expected to reach pre-1980 levels of ozone around the year 2068.
Acid Rain
Pure water measures 7.0 on the pH scale, meaning it is neither acidic nor basic (alkaline). Rainwater is often slightly acidic, with a pH somewhere between 5.0 and 7.0. This is because carbon dioxide in the atmosphere mixes with water and lowers the pH. Acid rain refers to precipitation that is more acidic than normal, with a pH of less than 5.0. The chemicals that cause acid rain come from both natural sources, such as volcanoes and decaying vegetation, and human-made sources, primarily emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) resulting from fossil fuel combustion. Acid rain occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds. The result is a mild solution of sulfuric acid and nitric acid. When sulfur dioxide and nitrogen oxides are released from power plants and other sources, prevailing winds blow these compounds across state and national borders and can cause acid rain far from the point of emission, sometimes over hundreds of miles away (Figure 12).
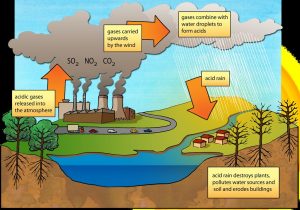
The ecological effects of acid rain are most clearly seen in aquatic, or water, environments, such as streams, lakes, and marshes. Most lakes and streams have a pH between 6 and 8, although some lakes are naturally acidic even without the effects of acid rain. Acid rain primarily affects sensitive bodies of water, which are located in watersheds whose soils have a limited ability to neutralize acidic compounds (called “buffering capacity”). Lakes and streams become acidic (i.e., the pH value goes down) when the water itself and its surrounding soil cannot buffer the acid rain enough to neutralize it. In areas where buffering capacity is low, acid rain releases aluminum from soils into lakes and streams; aluminum is highly toxic to many species of aquatic organisms.
Acid rain also causes slower growth, injury, or death of forests. Of course, acid rain is not the only cause of such conditions. Other factors contribute to the overall stress of these areas, including air pollutants, insects, disease, drought, or very cold weather. In most cases, in fact, the impacts of acid rain on trees are due to the combined effects of acid rain and these other environmental stressors.
Acid rain and the dry deposition of acidic particles contribute to the corrosion of metals (such as bronze) and the deterioration of paint and stone (such as marble and limestone). These effects significantly reduce the societal value of buildings, bridges, cultural objects (such as statues, monuments, and tombstones), and cars (Figure 13).

The Clean Air Act
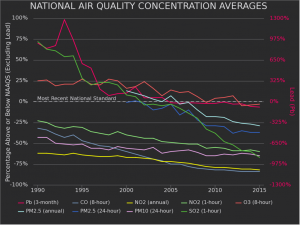
Government regulation has been one of the most effective methods of reducing air pollution. The Clean Air Act was passed in the United States in 1970 to regulate air pollutants such as lead, particulate matter, and carbon monoxide. The law was amended in 1990 to control acid rain-causing sulfur dioxide and nitrogen oxides as well. Due to the implementation of this law and further regulation, emissions of several major air pollutants have fallen since 1990 and are currently below levels that are considered unsafe (Figure 14). The EPA estimates that this reduction in air pollution prevents over 200,000 premature deaths and about 17 million lost work days per year. It has also found that the economic benefits of the Clean Air Act have been more than 30 times greater than the costs of implementing it.
Knowledge Check
Attribution
Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0. Modified from the original by Matthew R. Fisher and Sean Whitcomb.
Media Attributions
- US_primary_energy_consumption_by_source.svg © U.S. Energy Information Administration is licensed under a Public Domain license
- Oil_spill Gulf of Mexico © NASA is licensed under a Public Domain license
- Coal_power_plants_map © U.S. Dept. of Energy is licensed under a Public Domain license
- Coal_waste © Flashdark is licensed under a Public Domain license
- Hydraulic_Fracturing-Related_Activities © U.S. EPA is licensed under a Public Domain license
- Fukushima nuclear disaster © IAEA is licensed under a CC BY-SA (Attribution ShareAlike) license
- Hoover_Dam © U.S. Dept. of the Interior is licensed under a Public Domain license
- Solar_ASU © Kevin Dooley is licensed under a CC BY (Attribution) license
- Wind_turbines © amanderson2 is licensed under a CC BY (Attribution) license
- Ozone_layer © NOAA is licensed under a Public Domain license
- Ozone hole © NASA is licensed under a Public Domain license
- Acid_rain © Siyavula Education is licensed under a CC BY (Attribution) license
- Acid_rain_damage © Nino Barbieri is licensed under a CC BY-SA (Attribution ShareAlike) license
- National_Air_Quality_Concentration_Averages_1990-2015_US_EPA © U.S. EPA is licensed under a Public Domain license

