14 ANTIBIOTIC SENSITIVITY TESTING AND EFFECTIVENESS OF ANTISEPTICS AND DISINFECTANTS
LEARNING OBJECTIVES
Perform the Kirby-Bauer sensitivity test and evaluate the results
Differentiate between the Kirby-Bauer test and the Minimal Inhibitory Concentration test
Explain the difference between a disinfectant and antiseptic
MCCCD OFFICIAL COURSE COMPTENCIES
Describe and compare the effectiveness of physical and chemical methods of microbial control
Identify structural characteristics of the major groups of microorganism
Compare and contrast prokaryotic cell and eukaryotic cells
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Utilize aseptic technique for safe handling of microorganisms
MATERIALS
Media:
Mueller-Hinton Agar plate – 3/table
Antibiotic disks
Large TSA plate – 1/table
Selected disinfectants and antiseptics
Equipment:
Sterile swabs
Tweezers
Metric rulers
Stock Cultures:
Broth culture of Gram-negative Escherichia coli
Broth culture of Gram-negative Pseudomonas aeruginosa
Broth culture of Gram-positive Staphylococcus epidermidis
Class Demonstration:
Beta-Lactamase Test
BIOCHEMICAL TEST ALBUM
Antibiotics are chemicals produced by some bacteria and fungi that, in small quantities, can inhibit the growth of bacteria. Much of the success we have achieved in treating infections since World War II is due to the discovery of antibiotics. Utilizing the information gleaned from studying these natural chemicals, scientists have artificially synthesized other useful antimicrobial chemicals in the laboratory. Sometimes these antimicrobials are completely synthesized in the lab (synthetic) and sometimes they are partly produced in nature and partly synthesized in the lab (semisynthetic).
Many microbes can produce antibiotics, but four genera produce most of the antibiotics used for treating human and animal infections. Bacillus and Streptomyces are bacteria. Penicillium and Cephalosporium are fungi. It is a constant challenge to develop new antibiotics to replace those antibiotics for which microbes have developed resistance.
The range of bacteria killed by an antibiotic determines its “spectrum of activity”. Antibiotics that are only effective against Gram-positive or only effective against Gram-negative bacteria have a narrow spectrum of activity. Antibiotics that are effective against many different types of bacteria are called broad spectrum antibiotics. Broad spectrum antibiotics are probably contributing to the escalating drug resistance we are seeing in microorganisms. Broad spectrum antibiotics often wipe out a person’s normal microbiome as well as the pathogen they are intended to kill, resulting in superinfections from organisms such as Candida albicans and Clostridium difficile that grow out of control when they do not have to compete with microbes in the normal microbiomes.
Empiric therapy takes place when an antimicrobial agent is given to the patient without performing a culture or other diagnostic test to determine the specific cause of the disease. Empiric therapy is prescribed in instances where the causative pathogen is likely and where diagnostic tests will not change the treatment. The selection of which drug to use is based solely on experience, observation and relevant clinical information including current resistance patterns in suspected pathogens. These antibiotics are typically broad-spectrum, in that they treat a wide variety of possible microorganisms. Examples of this include antibiotics prescribed for strep throat, pneumonia, urinary tract infections, and suspected bacterial meningitis in newborns aged 0 to 6 months.
Physicians are beginning to target infections with narrow spectrum antibiotics, or synergistically treat infections with small doses of multiple antibiotics to try to prevent antibiotic resistance.
The laboratory can aid the physician in selecting which antimicrobial agent is likely to kill the pathogen that is causing an infection in a patient. There are several methods that are used by clinical microbiologists in this determination, including the Kirby-Bauer Test and the Minimal Inhibitory Concentration (MIC) Test.
Kirby-Bauer Test
The Kirby-Bauer test, known as agar disk diffusion, must be strictly regulated for the results to be interpreted correctly. Such characteristics as the stability of the antibiotic, the rate of diffusion of the antibiotic, the bacteria being tested, the pH of the culture medium, the depth of the culture medium, the inoculum density, the incubation time, the incubation temperature, and the concentration of the antibiotic can affect the results. Nevertheless, when the Kirby-Bauer test is performed under standardized conditions (on Mueller-Hinton agar inoculated with a pure culture of microbes that is the correct turbidity to match McFarland 0.5 standard, etc.) and the results are interpreted according to the Interpretative Zone Standards published by the National Committee for Clinical Laboratory Standards, the results provide an accurate prediction of which antibiotics are likely to be effective against the pathogen. Because the Kirby-Bauer test is relatively simple to perform and is inexpensive, it has been extensively used in medical practice. We will be performing a simplified version of the Kirby-Bauer Test in lab that is not standardized but will allow you to learn the general principles involved in this procedure. The results are reported as S (sensitive), I (intermediate), and R (resistant) to an antibiotic. A sensitive result indicates the bacteria will die when it is exposed to the antibiotic. An intermediate result indicates the antibiotic must be used in combination with another antibiotic to clear the infection. A resistant result indicates the antibiotic does not kill the bacteria.
The Minimal Inhibitory Concentration (MIC) Test
The MIC method determines the lowest concentration of an antibiotic that can inhibit the growth of a test organism. Although both the Kirby-Bauer and the MIC are accurate, the MIC gives a more precise indication of the dosage of the antibiotic that should be effective in controlling the infection in the patient. If that dosage of antibiotic can be achieved at the body site of infection (blood, urine, kidneys, etc.), the physician can determine the necessary dosage schedule and the route of administration. Generally, a margin of safety of at least ten times the MIC is desirable to ensure successful treatment of a disease.
An E test is a combination of the MIC and Kirby-Bauer Tests. A plastic-coated strip rather than a filter paper disk contains a gradient of antibiotic concentrations. These diffuse from the strip in the same way as the antibiotics diffuse into the media from the antibiotic containing filter-paper disks. Because there is a gradient concentration, the zone of inhibition will be different along the strip. A technologist can read the MIC from a scale that is printed on top of the disk.
Disinfectants and Antiseptics
Various chemicals can be used to control the growth of microbes outside of the body. There are many products on the market today that claim to kill bacteria, but how do you really know if they are doing the job they say they can do? To be a wise consumer, you need to define the terms found on the labels of these products. What does it mean to sterilize, disinfect or act as an antiseptic?
For a surface to be sterile there must be destruction of all life forms: vegetative cells, endospores and viruses. The microorganisms are either destroyed or physically removed from the objects or substances. Sterilization can be easily accomplished with the use of an autoclave or incineration, but rarely do any chemicals available in the marketplace have this ability.
The destruction of pathogens on inanimate objects is the job of a disinfectant. These chemicals rarely achieve total killing of all life forms, but they should be able to eliminate vegetative forms of most potentially hazardous and pathogenic microorganisms. This does not include endospores, tubercle bacteria and many viruses. The use of these chemicals is strictly limited to inanimate objects such as tabletops, tile walls, etc.
If a chemical is given the term antiseptic, it will be able to inhibit or destroy microorganisms, but not cause harm to living tissue. Antiseptics are less antimicrobial than other chemicals used as disinfectants. Some chemicals can be used as both a disinfectant and an antiseptic depending on their concentration.
PRE-ASSESSMENT
PROCEDURE
For this exercise: Each person at the table use one of the stock cultures:
Broth culture of E. coli (Gram-negative)
Broth culture of Pseudomonas aeruginosa (Gram-negative)
Broth culture of Staphylococcus epidermidis (Gram-positive)
Directions for the Kirby-Bauer Sensitivity Testing:
1. Three students at a table, obtain a Mueller-Hinton agar plate, sterile swab and a bacterial broth culture.
2. Label the agar side of the plate with your name, table and the name of your bacteria. Determine if your bacterium is Gram-positive or Gram-negative. This information will dictate which antibiotics you will test.
3. Suspend the bacteria in a broth culture by gently shaking the tube side to side until the bacteria swirl up from the bottom of the tube.
4. Use a sterile cotton-tipped swab to obtain the inoculum. Peel the paper cover from the wooden end of the swab. Handle ONLY the wooden end of the swab to maintain the sterility of the cotton end.
5. Remove the cap of the bacterial culture with your little finger. Dip the cotton swab into the broth and then slightly press the swab against the inside of the tube to expel any excess broth.
6. Streak the Mueller-Hinton agar with the swab to achieve confluent growth (growth over the entire agar surface). To obtain confluent growth, swab the entire surface of the agar. Do NOT dip the swab into the broth again. Rotate the plate 90 degrees and swab again evenly over the entire plate. Rotate the plate 90 degrees one final time and swab the plate evenly again.
7. Dispose of the swab in the autoclave bag and allow the plate to dry for 3 minutes.
8. Dispense an antibiotic disk from each cartridge assigned to the Gram-positive and/or Gram-negative bacteria. (E. coli and Pseudomonas are Gram-negative and Staphylococcus epidermidis is Gram-positive).
9. Take the tweezers out of the alcohol and let it evaporate.
10. Use the sterile tweezers to gently press the disks to contact the media.
11. Invert the plate and incubate it on the class plate tray until the next lab session.
AFTER INCUBATION
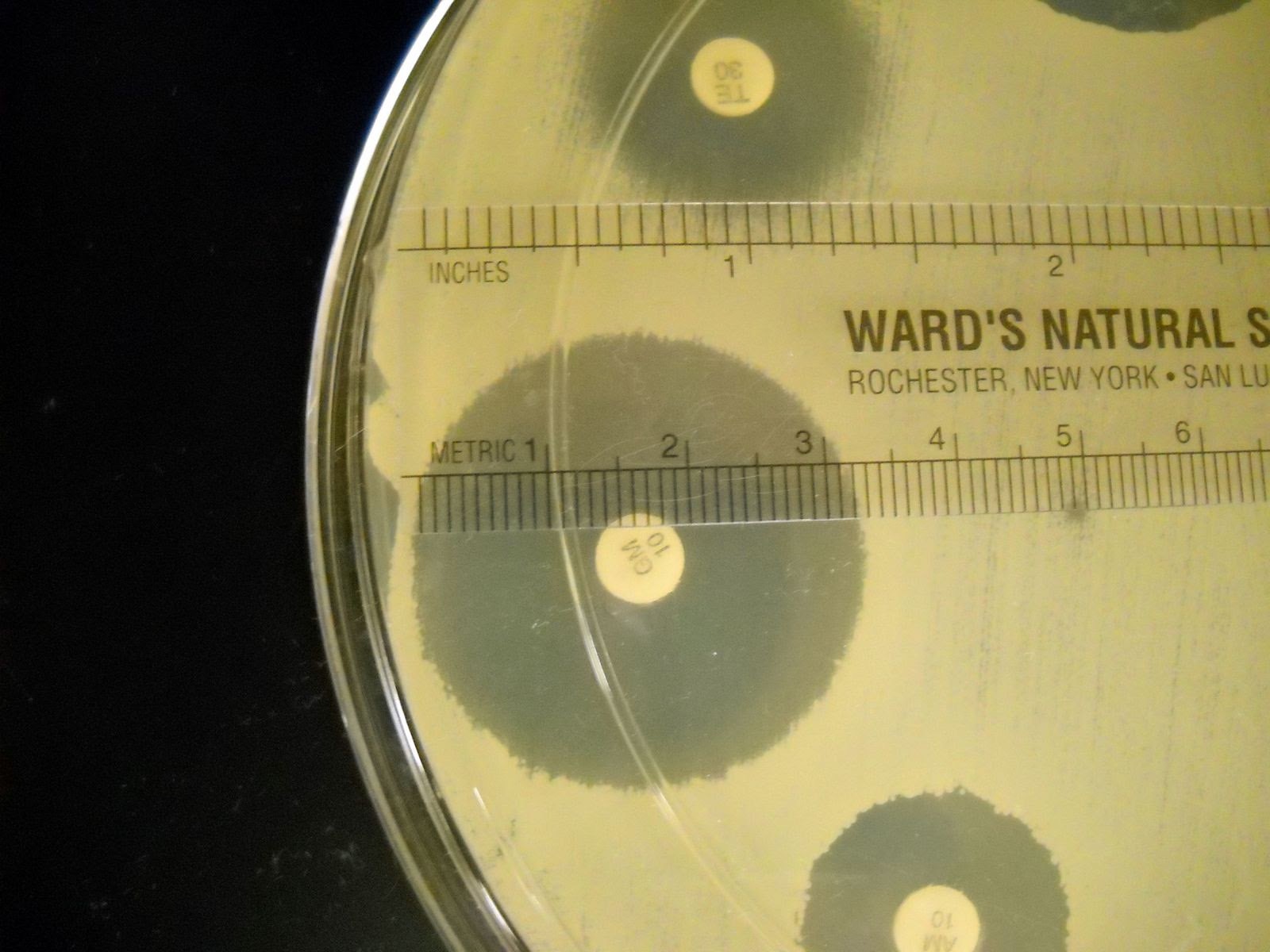
1. Measure the diameter of each zone of inhibition for each disk in millimeters. Record the results on the worksheet.
- Measure the diameter of the zone of inhibition from one edge to the other; right over the middle of the antibiotic disk.
- If the bacteria grow right up to the disk, the zone of inhibition is reported as “6 mm.” which is the diameter of the disk. This indicates that the bacteria are resistant to that antibiotic.
- If mutant strains are growing inside the general zone of inhibition or if the edges of the zones are “fuzzy”, measure the innermost clear zone.
2. The zone of inhibition is the result of two factors: how quickly the organism grows and how fast the antibiotic diffuses through the media. Since antibiotics have different molecular weight and therefore different diffusion rates, antibiotics cannot be compared to each other. Instead, each antibiotic zone size must be compared to a standardized chart.
3. Compare the zone sizes of your zones to the standardized charts on the worksheet. Determine of the bacteria is sensitive, intermediate, or resistant to each antibiotic. Note that each bacterium has a separate chart.
4. Record your results on the worksheet.
Directions for determining the effectiveness of disinfectants/antiseptics:
1. Assign one of the stock organisms to one person at the table. Obtain a TSA plate, sterile swab and the assigned test organism. Label the TSA plate with the bacteria name and your name and table.
2. Divide the plate into eight sections (pie pieces) and label each section with the antiseptics or disinfectants to test.
3. Suspend the bacteria in a broth culture by gently shaking the tube side to side until the bacteria swirl up from the bottom of the tube.
4. Use a sterile cotton-tipped swab to obtain the inoculum.
5. Streak the T. soy agar with the swab to achieve confluent growth (growth over the entire agar surface). To obtain confluent growth, swab the entire surface of the agar. Do NOT dip the swab into the broth again. Rotate the plate 90 degrees and swab again evenly over the entire plate. Rotate the plate 90 degrees one final time and swab the plate evenly again.
6. Dispose of the swab in the autoclave bag and allow the plate to dry for 3 minutes.
7. Take the tweezers out of the alcohol and let it evaporate.
8. Using the tweezers, pick up a sterile paper disk and fully dip it into a disinfectant or antiseptic of choice and then slightly press the disk against the inside of the beaker to expel any excess liquid. Place the disk on the appropriately labeled section of the inoculated media.
9. Repeat the procedure with the other chemicals, using 8 chemicals on the plate.
10. Use the sterile tweezers to gently press the disks to contact the media.
11. Invert the plate and incubate it on the class plate tray until the next lab session.
AFTER INCUBATION
1. Measure the diameter of the zone of inhibition for each chemical in millimeters. If there is no zone around the disk, record it as 6mm (the diameter of the disk).
2. Record the results on the worksheet.
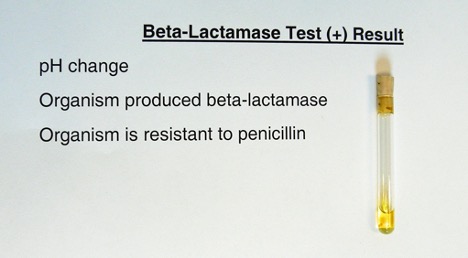
Demonstration of Beta-Lactamase Activity
Determine if one of the organisms you tested can synthesize the enzyme beta-lactamase. Beta-lactamase will destroy the beta-lactam ring of penicillin. The end product will be penicilloic acid.
1. The instructor will add approximately 5 drops of deionized water to the tube containing penicillin and a pH indicator. Using a sterile, cool inoculating wire, a heavy inoculum will be used to make a suspension of organisms in the tube.
2. Observe the tube for a color change for two minutes.
Yellow = Positive (due to the acidic pH change)
Pink = Negative
Answer a few questions about the demonstration on the worksheet.

POST TEST
DISCOVERIES IN MICROBIOLOGY

DR. JANE HINTON
American veterinarian Dr. Jane Hinton helped create Muller Hinton agar in 1941. It is non-selective, non-differential medium, so almost all bacteria can grow on it. The starch in the media absorbs toxins released from the bacteria, so they do not interfere with the antibiotic. It is a loose agar; this allows for better diffusion of the antibiotics than most other plates. A better diffusion leads to a more accurate zone of inhibition.

