4 PARASITES: PHYLUM PLATYHELMINTHES
LEARNING OBJECTIVES
Identify representative platyhelminth pathogens
Describe life cycles and unique characteristics of representative flatworms
MCCCD OFFICIAL COURSE COMPETENCIES
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cells
Compare and contrast the physiology and biochemistry of various groups of microorganisms
Describe the symptoms, associated pathogen, transmission, course, treatment and prophylaxis of common infectious diseases
Apply various laboratory techniques to identify types of microorganisms
MATERIALS
Microscope Demonstrations of:
Clonorchis sinensis adult fluke in liver tissue – 50X total magnification
Clonorchis sinensis ova – 400X total magnification
Schistosoma mansoni ova – 400X total magnification
Schistosoma mansoni cercariae – 100X total magnification
Schistosoma mansoni adult (male & female) – 50X total magnification
Taenia saginata ova – 400X total magnification
Echinococcus granulosus hydatid sand – 400X total magnification
Echinococcus granulosus Adult – 50X total magnification
Demonstrations of Preserved Specimens of:
Clonorchis sinensis adult
Taenia saginata adult
PARASITE ALBUM LINK
Platyhelminthes are commonly known as flatworms. There are two classes of flatworms: Trematoda, commonly called flukes, and Cestoda, known as tapeworms. All Platyhelminthes described here have a complex life cycle, involving at least one intermediate host in which the parasite must mature before becoming an adult worm. The animal that harbors the adult worm is called the definitive host.
Class Trematoda – flukes
Trematodes (flukes) are non-segmented, leaf-shaped parasites that have a simple digestive tract and require at least one intermediate molluscan host (e.g. snail) in their life cycle. Flukes are named according to the host tissue in which the adults live (e.g. liver, blood, lung, or intestinal fluke). The two flukes described in this lab are Clonorchis sinensis, whose common name is the Chinese liver fluke, and Schistosoma, commonly called the blood fluke.
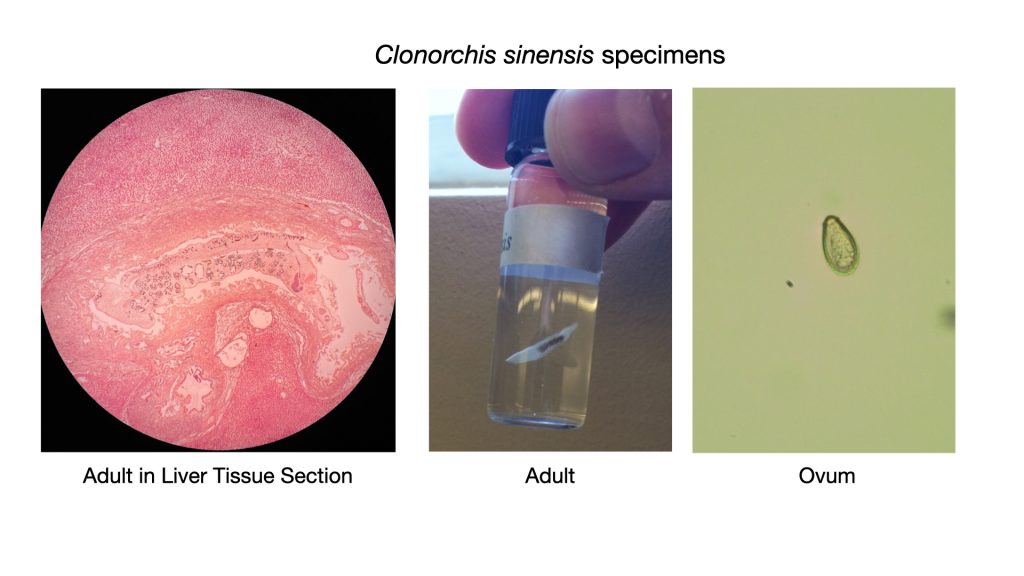
Clonorchis sinensis (klo-nor’-kis/sin-en-sis)
An infected host sheds Clonorchis ova (eggs) in the feces. If the feces gain access to water, the eggs will develop into miracidium larvae which may be ingested by a freshwater snail. The parasite will mature in the snail and emerge as free-swimming cercaria (hundreds of cercaria may be released) which will then penetrate the flesh of freshwater fish. As a human eats the infected fish raw or undercooked, the metacercaria is released in the human intestines. A worm emerges which migrates up the bile ducts to the liver. Clonorchis is a monoecious organism. This means both sexes occur in a single animal. One adult Clonorchis contains both male and female reproductive organs. It can thus fertilize itself.
The laboratory diagnosis of Clonorchis infection is made by identifying the characteristic small, pumpkin seed shaped ova in stool specimens. The ovum has a prominent convex operculum that appears to rest on top of the egg.
Clonorchis has been known to cause biliary obstruction and jaundice. It may also cause intermittent diarrhea, liver pain, anemia, and abdominal distress. Praziquantel has proven to be an effective treatment.
Infections with Clonorchis are most common in the Far East, particularly in Vietnam, Japan, Korea, Formosa and southern China. Currently, it is estimated that over 200 million people are at risk of C. sinensis infection, and around 15 million are infected China, South Korea, northern Vietnam, and far east of Russia. These countries make use of “night soil” (human waste) as an economical, nutrient rich fertilizer, especially for rice paddies. In addition, the people in these cultures frequently consume raw fish in their diet. Only thorough cooking of freshwater fish can eliminate this disease in humans. Refrigeration, salting, smoking or adding vinegar to fish is insufficient to kill Clonorchis.
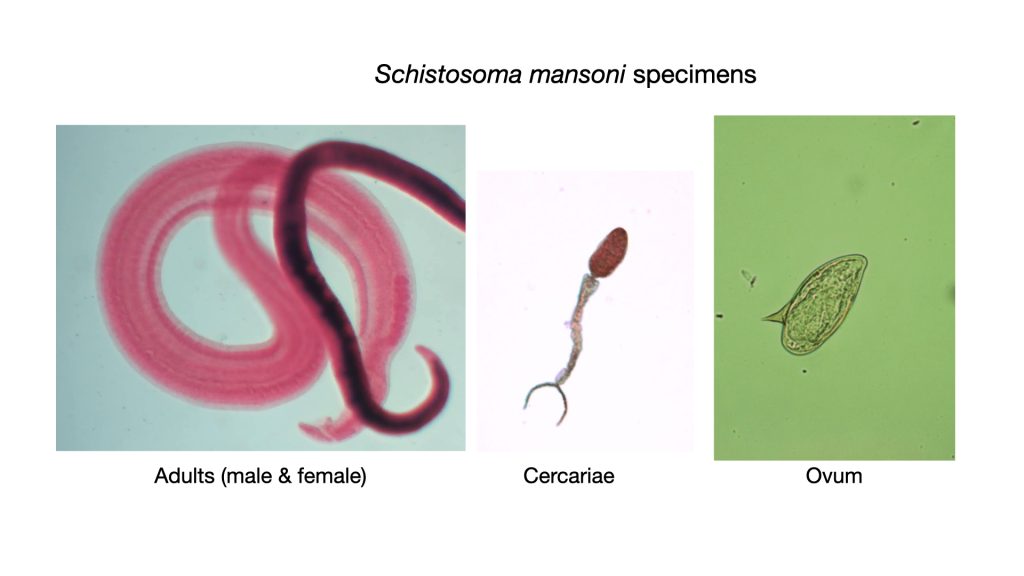
Schistosoma mansoni (shis’to-so’muh/man-so’nigh)
There are several species of Schistosoma that cause disease in man. Each species is unique to a particular area in the world. Since the United States does not have the specific host snails needed for its life cycle, infections are not contracted directly, yet many immigrants and travelers to endemic areas are infected. An estimated 236 million people require preventative drug treatment per year, out of which 105 million people were reported to have been treated. Preventative treatment, which should be repeated for a number of years, will reduce and prevent morbidity.
Individuals infected with Schistosoma excrete the ova in the feces. Upon contact with water, the ova (which look like a cartoon talk bubble) develop into miracidia which enters the snail. Maturation in the snail results in the production of the free swimming cercaria. When the cercaria contacts the skin of a person who is in the water, it secretes enzymes that enable it to burrow through unbroken skin. The Schistosoma are then carried in the bloodstream to the liver or urinary bladder where they mature into an adult. Adults can live up to seven years. Schistosoma is dioecious – the male and female are separate animals. If both male and female are present, fertilization occurs, and ova are produced. The cycle then continues in a new host. Schistosoma infection may result in destruction of the liver, lungs, and/or urinary system. This is second most devastating parasitic disease. (Malaria is the most common). Praziquantel is an effective treatment.
Birds in the United States suffer from a Schistosoma infection. The Schistosoma parasite that infects birds has an intermediate snail host that is predominantly found in water in the Great Lakes and on the east coast. Sometimes when the free swimming cercaria of this bird parasite encounters a human swimming in the water, it will attempt to burrow through the skin of the human host instead of the normal bird host. Since humans are not the correct host for this parasite, the cercaria are unable to penetrate the human skin and instead, become embedded in the skin. This causes a painful, itchy inflammatory dermatitis called “swimmer’s itch”.
Class Cestoda – tapeworms
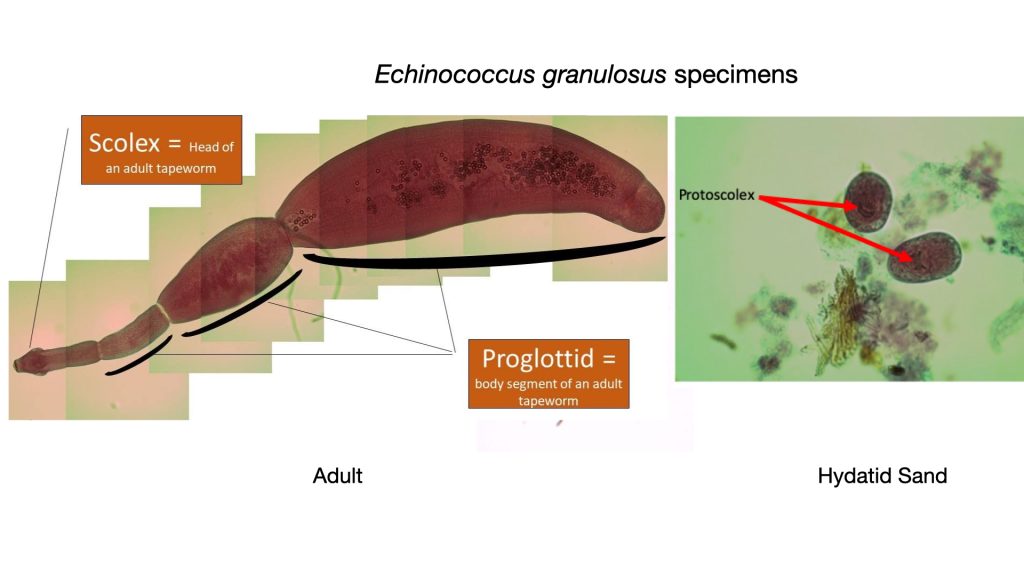
Tapeworms are segmented, ribbon-like flatworms that lack a digestive tract of their own. They must rely completely on the digestion of the host they inhabit. Adult tapeworms can live in the small intestine of the host where they attach themselves by suckers or hooks that are part of the scolex or head of the worm. Proglottids are body segments of the worm that extend from the head. It is in these proglottids that ova are produced. All tapeworms are monoecious-both ovaries and testes are in one animal. Praziquantel is an effective treatment for tapeworm infections.
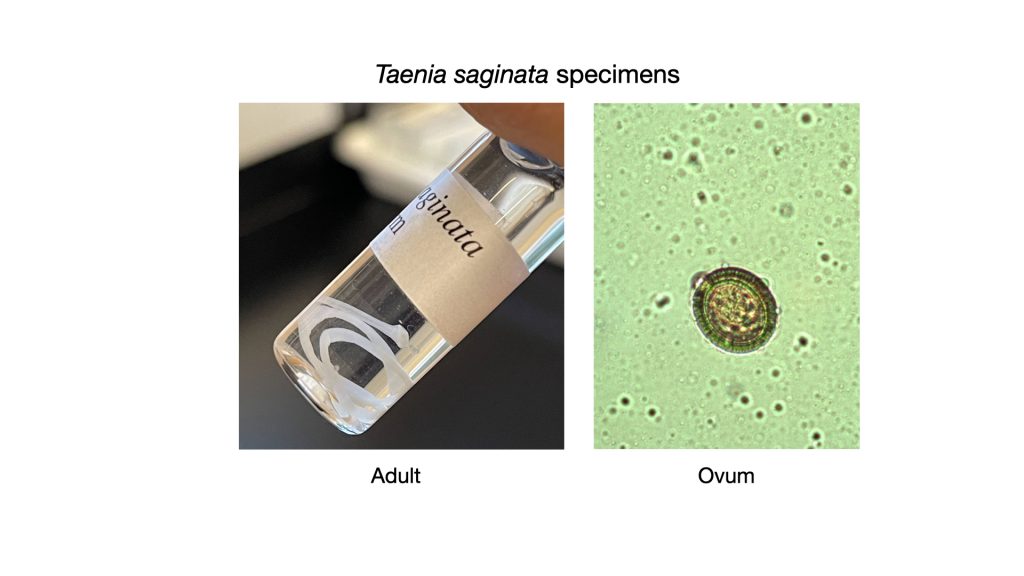
Taenia saginata (tee’nee-uh/sadj-i-nay’tah)
Taenia saginata has the common name “beef tapeworm” because the cow is the intermediate host of this parasite. The ova passed in the feces of the human host harboring the adult tapeworm contaminate soil or water where they can be ingested by cows. The ova hatch in the cow’s intestines, liberating larvae that find their way into the skeletal muscle of the cow. In the muscle, the larvae become encysted in a form called cystercerci, or “bladder worms”. Humans become infected by eating raw or insufficiently cooked beef. The cystercerci in the beef enter the human intestine where a scolex everts and attaches to the intestinal mucosa. The worm begins to grow, elongating as proglottid segments are added. Ova are produced in the proglottids and shed in the feces.
Adult Taenia saginata may reach a length of 10 meters and live up to 25 years. T. saginata infections are infrequently encountered in the United States now, partly due to public education about ingesting undercooked beef.
Taenia solium (tee’nee-uh/so-lee’um)
Taenia solium has the common name “pork tapeworm”. The life cycle of T. solium is like the life cycle of T. saginata except that the intermediate host is the pig. People get infected by ingesting the larvae when eating raw or undercooked pork. Most symptoms are mild or nonexistent, but it may cause abdominal pain, loss of appetite and weight loss.
The more severe disease caused by Taenia solium is cysticercosis. Humans ingest T. solium ova from contaminated soil and become the host of the larval form of T. solium. The larvae (cystercerci) develop and migrate to the brain and develop cysts. Symptoms may mimic those of a brain tumor. Infection with T.solium is prevalent in certain parts of Mexico and Central America. It is also found in the United States, predominantly in Hispanic cultures and in people who have traveled to areas where the parasite is commonly found.
Tapeworm infections are diagnosed by finding ova in the feces. These ova are spherically shaped and covered with a thick striated shell (looking similar to poker chips). Because the ova of T. solium are indistinguishable from those of T. saginata, it is critical that extreme caution be exercised by health personnel when handling or disposing of feces from humans who have Taenia infections. The differentiation of T.solium from T. saginata relies on observation of the scolex or proglottids of the adult tapeworm. The scolex of T. solium has, in addition to four suckers, a double crown of prominent hooks. The scolex of T. saginata lacks hooks. The proglottids of T. saginata have more than fifteen lateral branches on each side of the uterus, while T. solium has eight to thirteen branches.
Echinococcus granulosus
Cystic echinococcosis (CE) is caused by infection with the larval stage of Echinococcus granulosus. CE is found in Africa, Europe, Asia, the Middle East, Central and South America, and in rare cases, North America. The parasite is transmitted to dogs when they ingest the organs of other animals that contain hydatid cysts. The cysts develop into adult tapeworms in the dog (definitive host). Hydatid cysts contain a structure called a protoscolex which develops into the adult tapeworms scolex when ingested. Infected dogs shed tapeworm eggs in their feces which contaminate the ground. Sheep, cattle, goats, and pigs ingest tapeworm eggs in the contaminated ground; once ingested, the eggs hatch and develop into cysts in the internal organs. The most common mode of transmission to humans is by the accidental consumption of soil, water, or food that has been contaminated by the fecal matter of an infected dog.
Most infections in humans are asymptomatic, but CE can cause slowly enlarging masses/cysts. When the masses grow large enough to cause discomfort, pain, nausea, and vomiting. The cysts are mainly found in the liver and lungs but can also appear in the spleen, kidneys, heart, bone, and central nervous system, including the brain and eyes. Cyst rupture is most frequently caused by trauma and may cause mild to severe anaphylactic reactions, even death, as a result of the release of cystic fluid.
Imaging techniques are used to detect hydatid cysts. These cysts are treated with a combination of medication and surgery.
PRE-ASSESSMENT
procedure
Draw microscope demonstrations and preserved demonstrations on the worksheet.
POST TEST
DISCOVERIES IN MICROBIOLOGY
DR. JULIUS PETRI
 German microbiologist Dr. Petri worked in Robert Koch’s lab in Germany. At the time, media was poured into an open dish and placed under a jar. The plate was exposed to the air each time the culture was viewed under a microscope or with the naked eye. In 1887 Petri had the idea of placing a slightly larger glass lid on top of the glass dish that contained the culture media. Petri’s dish was simple and reduced contamination. The design of the dish has not changed since it was invented.
German microbiologist Dr. Petri worked in Robert Koch’s lab in Germany. At the time, media was poured into an open dish and placed under a jar. The plate was exposed to the air each time the culture was viewed under a microscope or with the naked eye. In 1887 Petri had the idea of placing a slightly larger glass lid on top of the glass dish that contained the culture media. Petri’s dish was simple and reduced contamination. The design of the dish has not changed since it was invented.

