5 PARASITES: PROTOZOA
LEARNING OBJECTIVES
Identify representative protozoan pathogens
Describe life cycles and unique characteristics of representative protozoan pathogens
MCCCD OFFICIAL COURSE COMPETENCIES
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cells
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
Describe the symptoms, associated pathogen, transmission, course, treatment and prophylaxis of common infectious diseases
Apply various laboratory techniques to identify types of microorganisms
MATERIALS
Microscope Demonstrations of:
Trichomonas vaginalis trophozoites – 400X total magnification
Giardia lamblia trophozoites– 400X total magnification
Balantidium coli trophozoites and cysts – 400X total magnification
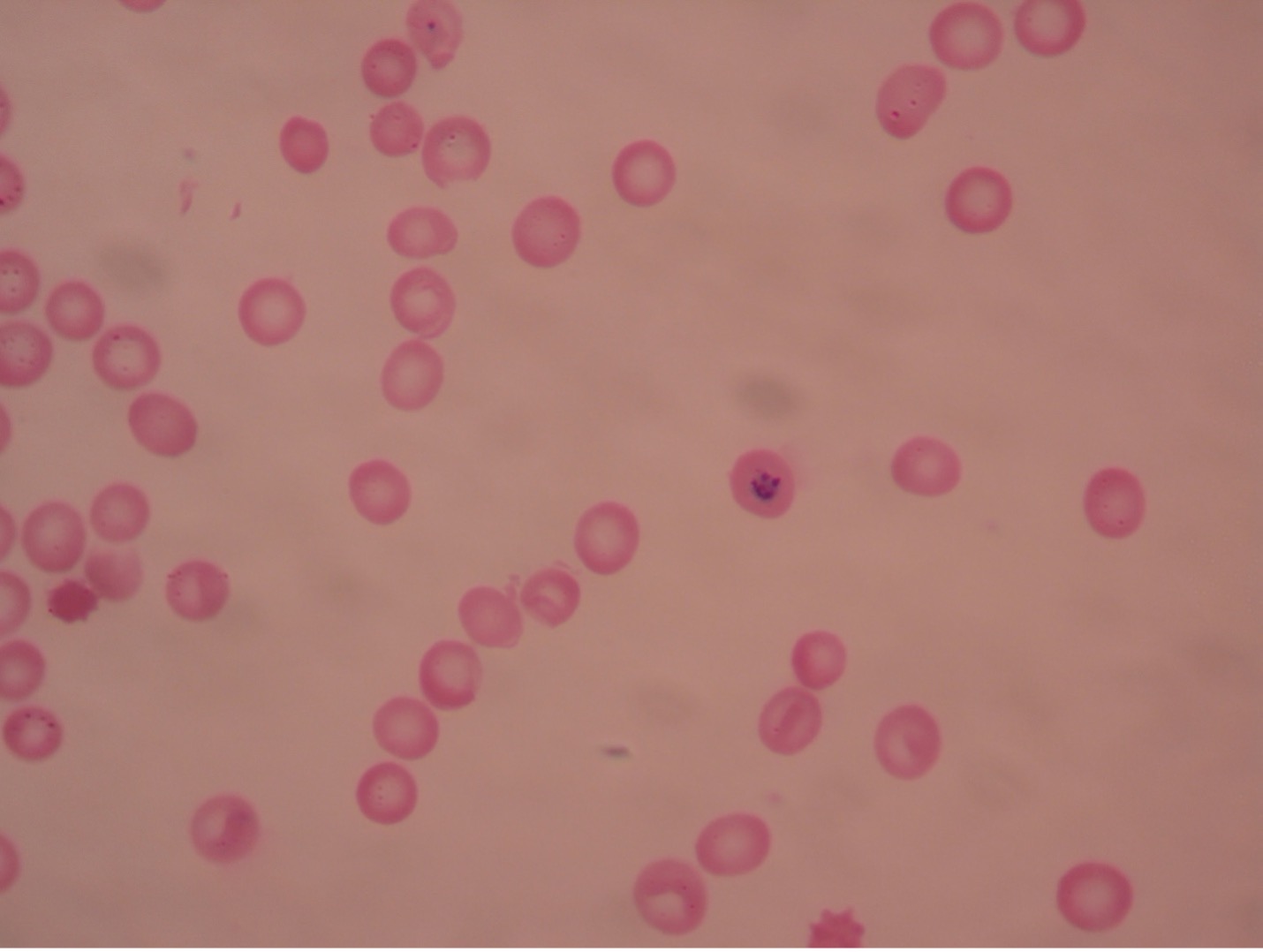
Plasmodium blood smear – 1000X total magnification
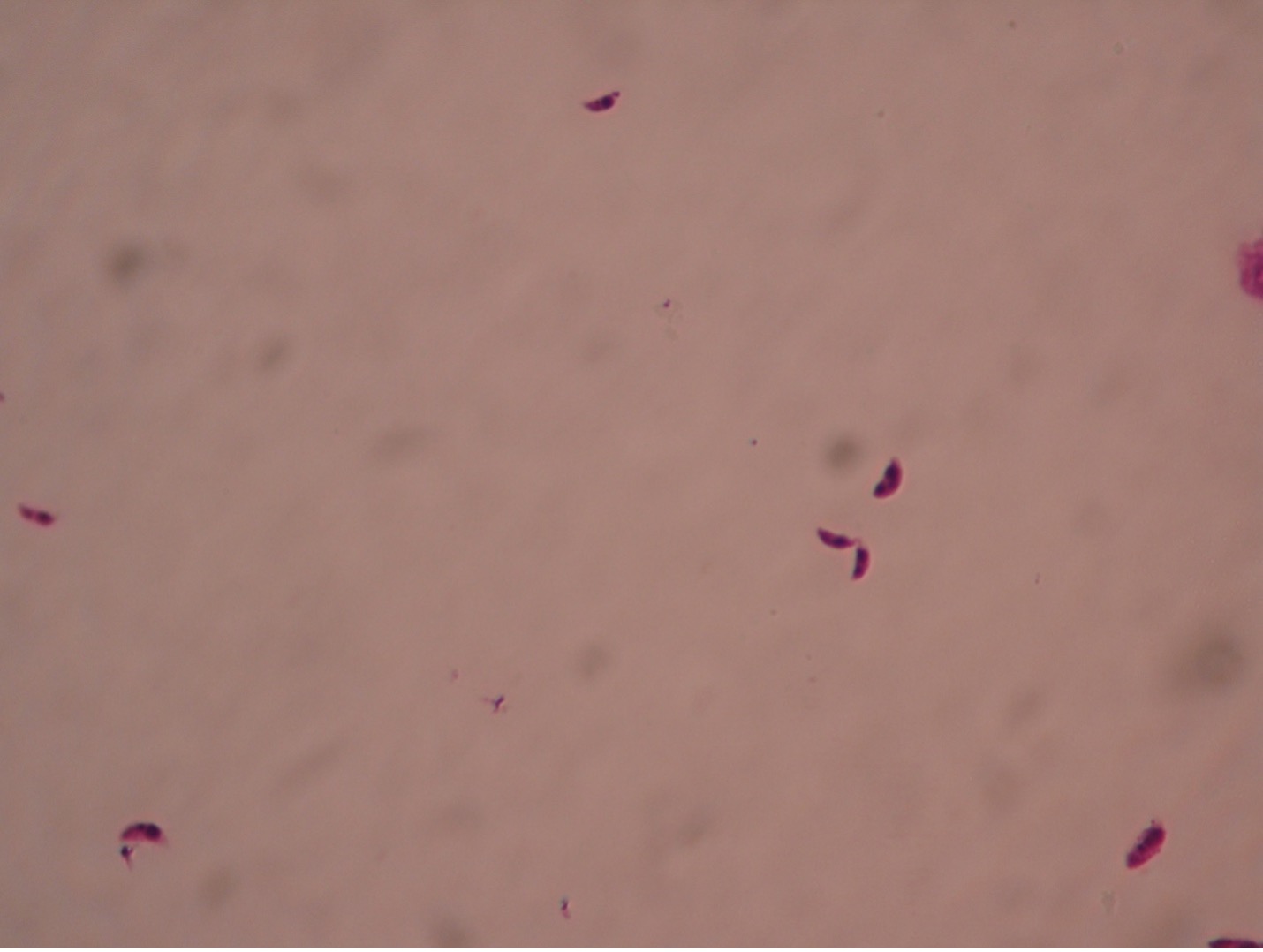
Trypanosoma blood smear– 1000X total magnification
PARASITE ALBUM LINK
All protozoans are found in a life stage called trophozoites. The “troph” stage is actively feeding, mostly motile and responsible for symptoms in a host. Some protozoans can form a cyst stage – a resting, inactive stage that protects the organism because of a thick wall that is produced. These cysts enable the protozoan to survive harsh environmental conditions outside of a host and ensure the organism will be passed to other hosts. Ingestion of the cyst in contaminated food or water is a common method of acquiring many protozoan infections.
Classification of Protozoans
Classification of microorganisms is dynamic, changing frequently as new information becomes available. Protozoa is not an official term used in the classification of microorganisms. Rather, it is a term used to group eukaryotic organisms that are unicellular and have no cell wall. These organisms are frequently organized into subgroups based on their means of locomotion. Some scientists refer to these locomotion groups as phyla; others refer to them as classes. We will just group these organisms according to their method of locomotion, so we have a common means of communicating when we are discussing these parasites.
Protozoans with Pseudopods
Entamoeba histolytica (en’tuh-mee’buh/his-toe-lit’i-kuh) move by extruding cytoplasmic extensions called pseudopods or “false feet”. Worldwide, approximately 50 million cases of invasive E. histolytica disease occur each year, resulting in as many as 100,000 deaths. This represents the tip of the iceberg because only 10%-20% of infected individuals become symptomatic. In the U.S. it is most commonly seen in travelers or immigrants from tropical countries with poor sanitary conditions. When traveling, drink bottled water or boil the water for 1 minute to destroy the parasite. Metronidazole (Flagyl) may be used to treat the infection.
Entamoeba causes the disease, amoebic dysentery or amoebiasis. Dysentery is characterized by bloody diarrhea which can result in severe dehydration. People become infected with Entamoeba by ingesting cysts in contaminated food or water. The trophs lie in the lumen of the large intestine. They invade the mucosal crypts, feed on red blood cells (red cells are often seen inside stained Entamoeba specimens). The symptoms are usually mild. Rarely, the organism may form intestinal ulcers. The ulceration of the intestinal wall causes dysentery symptoms (bloody stools containing pus and mucous). Entamoeba migrates through the blood to the liver where it may cause severe abscesses within 2-4 weeks.
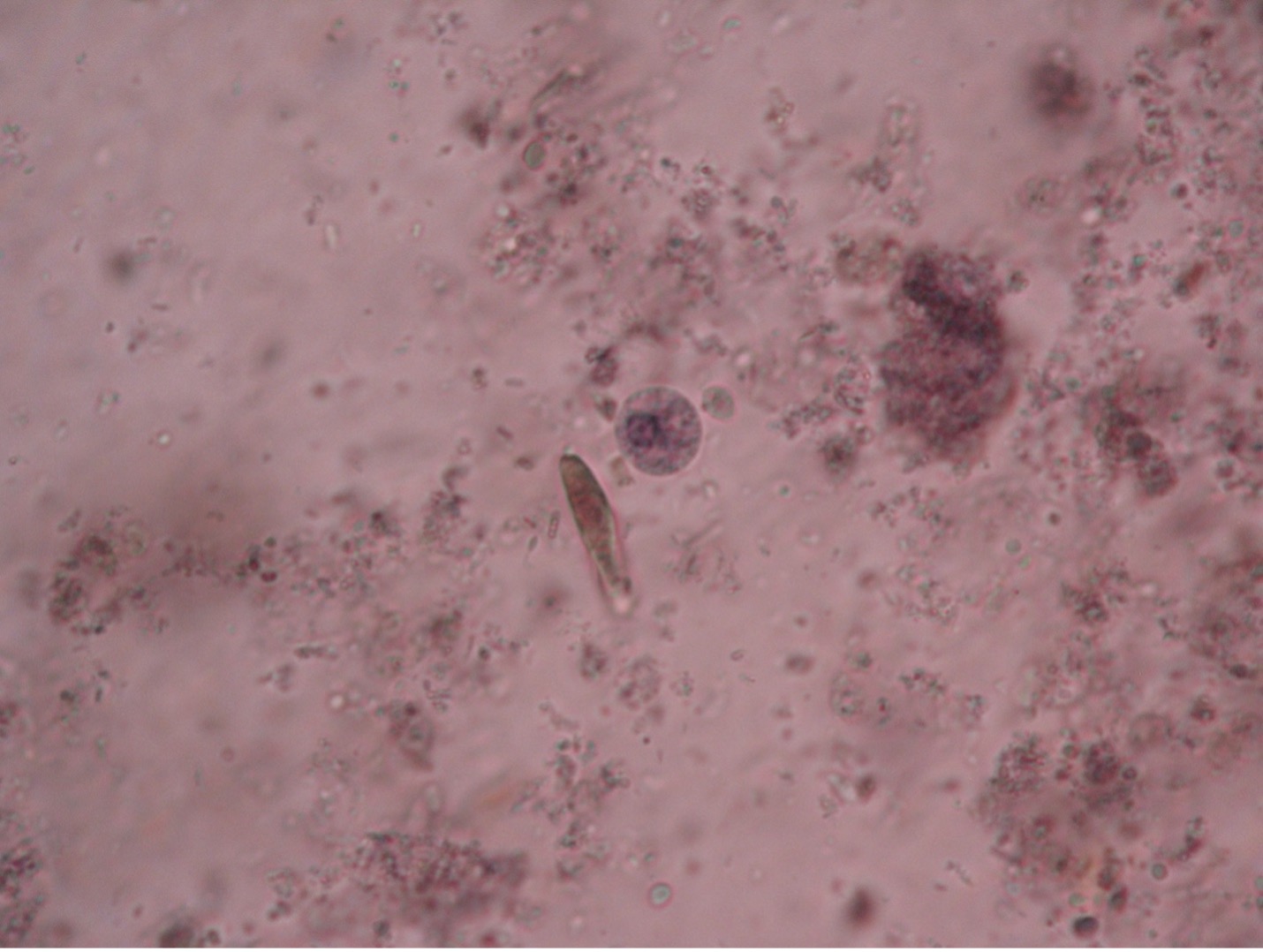
It is possible to differentiate trophozoites from cysts. Trophozoites are irregular in shape and pseudopods can be seen. The troph form has a single nucleus, while the cyst contains a maximum of four nuclei. Each nucleus may contain a dark staining dot called a karyosome. A cigar-shaped chromatin body may also be seen in either the cyst or troph stage. The cyst stage is notable for its nearly perfect spherical shape.
Naegleria fowleri (nay’gleer-ee’uh/fowl’-er-eye) is found in the sandy bottoms of warm water ponds, lakes, and hot springs. It gets stirred up when people jump or dive into shallow bodies of water. It is not found in salt water. It is most often observed in times of drought when water levels are low and water activities are more likely to stir up sand and dirt from the bottom of the pond or lake. Nose plugs are thought to help prevent infection. The organism is very invasive and if it gets high into the nasal passages, it can pass via the olfactory nerve into the brain where it sets up a fatal infection called primary amoebic meningoencephalitis (PAM). This destruction of brain tissue produces symptoms like bacterial meningitis such as headache, fever, nausea, vomiting and stiff neck, as well as seizures and hallucinations. Death usually occurs within three to seven days after the onset of symptoms despite treatment. Naegleria fowleri infections are rare. According to CDC, 33 infections were reported in the U.S. from 2011 to 2020.
Acanthamoeba (ay-kanth’uh-mee’buh) is a free-living freshwater amoeba that has been implicated in eye infections. Eighty-five percent of infections in the United States involve improper cleaning of contact lenses. The infection may cause permanent visual impairment or blindness. It can also cause invasive encephalitis in immunocompromised individuals. While it is still possible to become infected if one does not wear contact lenses, most cases can be prevented by storing and handling contact lenses appropriately – using proper cleaning solutions to clean lenses, washing hands before handling contact lenses, not swimming, using a hot tub or showering wearing lenses, wearing and replacing lenses according to the prescribed schedule, using clean solution to wash lenses, replacing storage cases at least once every three months, and getting regular eye examinations. Most people will be exposed to Acanthamoeba in their lifetime, but most will not become infected.
Protozoans with Flagella
Trichomonas vaginalis (trick’o-mo’nas/vadj-i-nay’lis) has no cyst stage so it cannot survive outside its host very long. It is transmitted by intimate contact between individuals. It is the cause of trichomoniasis, commonly called “ping-pong vaginitis” because it may be passed back and forth between sexual partners. In the United States, an estimated 2 million people have the infection, but only about 30% develop any symptoms of trichomoniasis. While most infections are asymptomatic, it can cause prostate and epididymis infections in men. Females report frequent urination, itching, burning, and a vaginal discharge. Diagnosis often occurs when people seek medical help for what they believe is a urinary tract infection. Flagyl is used to treat infections. Diagnosis is usually made by finding the tear-dropped shaped trophozoites in a wet preparation of a vaginal or urethral discharge. Three to five flagella may be visible as a tuft at the anterior end of the cell. T. vaginalis will exhibit a quick, jerky, darting motility as it zooms around the field of vision.
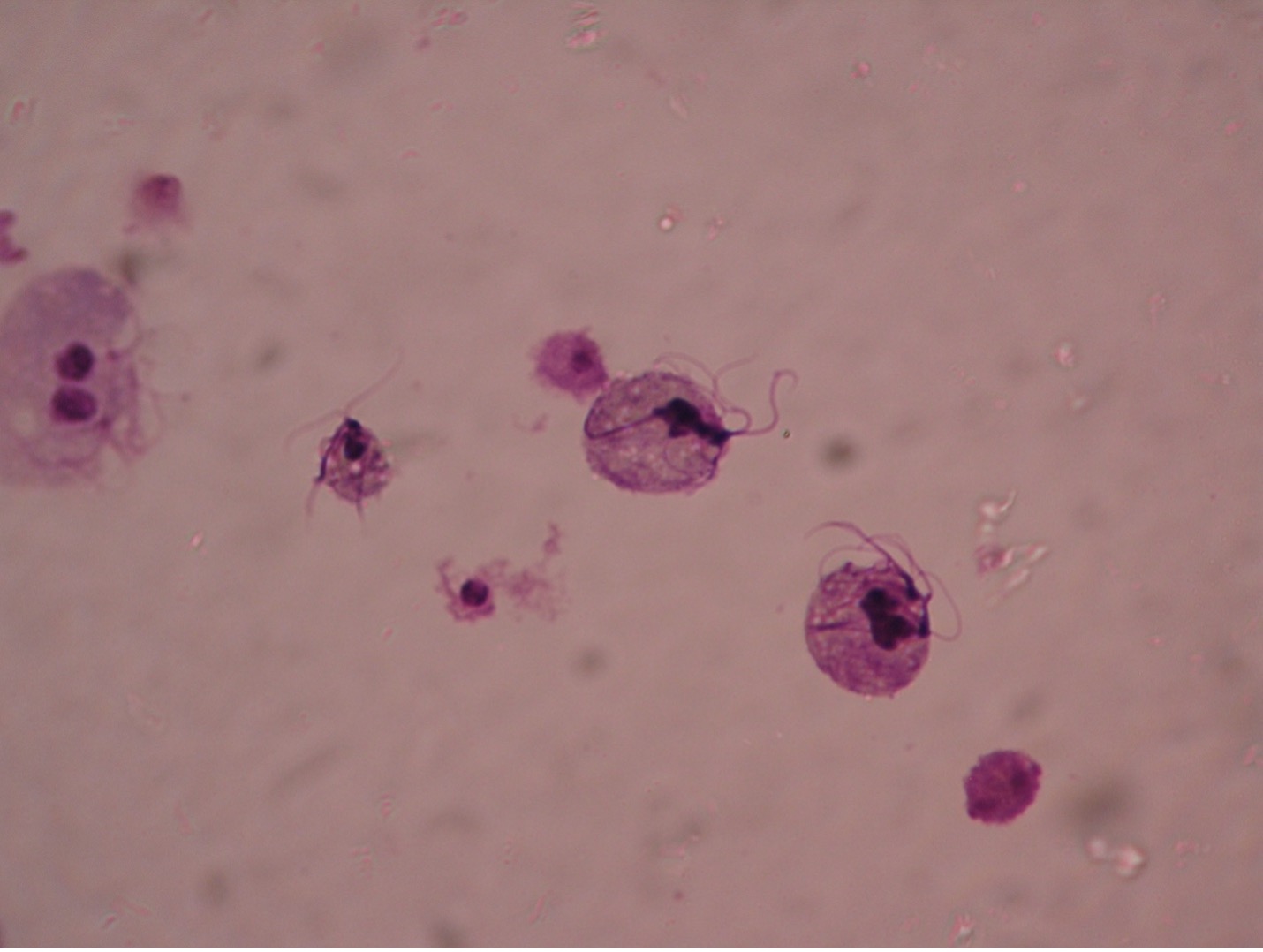
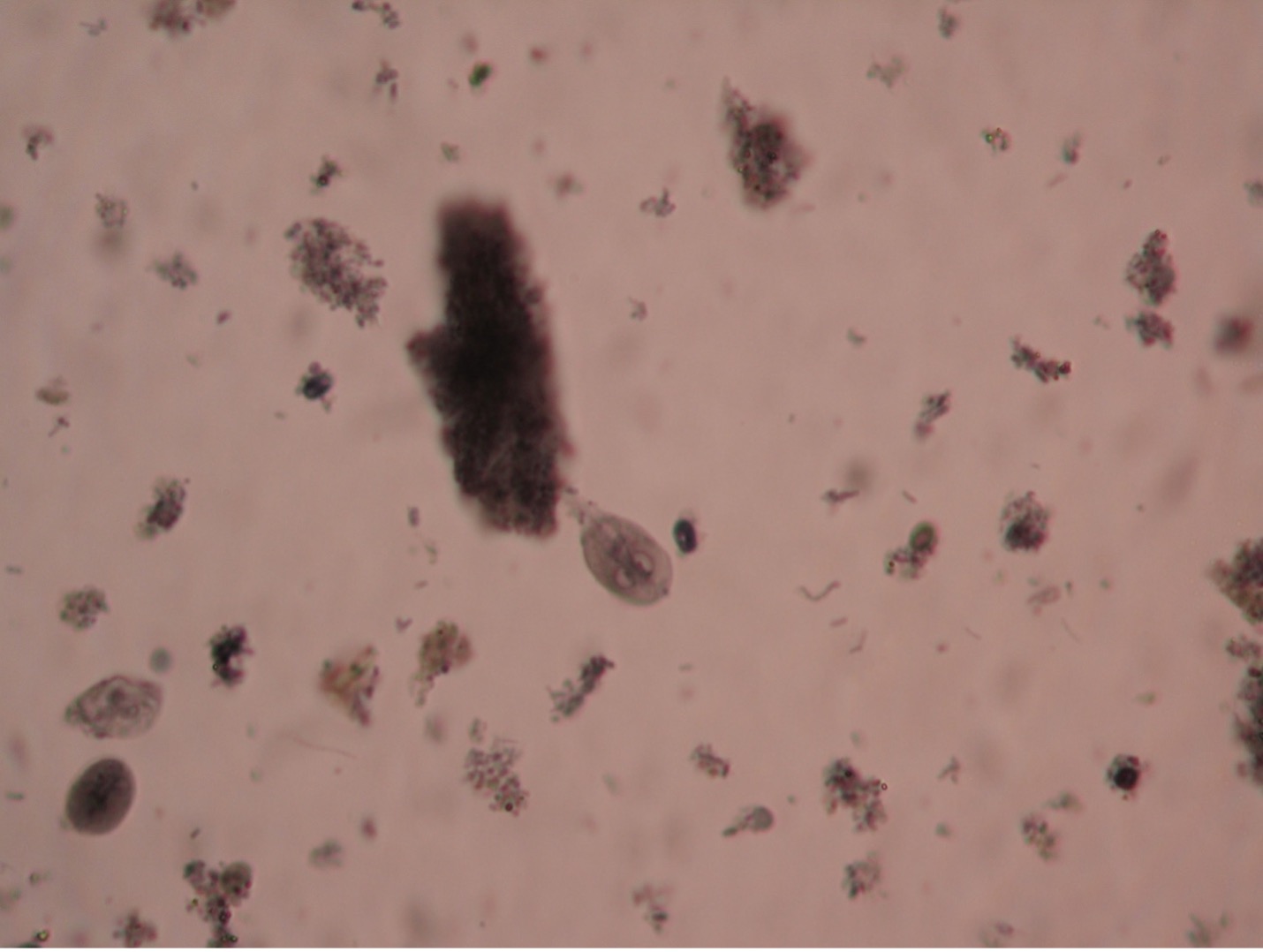
Giardia lamblia (gee’are-dee’uh/lamb-blee’uh) are unique in appearance in either the trophozoite or cyst stage. The troph is a pear-shaped flagellate with a broad, rounded anterior and a tapered posterior extremity. An oval, concave sucking disc occupies about three-fourths of the flat ventral surface which attaches to the mucosal lining of the hosts digestive tract. Two nuclei, each with a large central karyosome resembling “eyes on a face” help distinguish this parasite from other protozoans. Four to five pairs of flagella maybe present. The cyst stage is oval, and two to four nuclei can usually be observed.
The common name for Giardia is “backpacker’s parasite”, a result of the cases acquired by those who drink water without purifying it. Animals such a beaver, elk, and deer may carry the parasite and deposit cysts in streams when they defecate. The parasite is commonly found in cats, dogs, sheep, deer, and cows. When ingested by a new host the cysts pass unharmed through the gastric juices and undergo excystation in the duodenum. Therefore, trophs of Giardia are found in the upper part of the small intestine. Giardia infection is the most common intestinal parasitic disease in the United States infecting more than 1 million people per year. Symptoms often begin within 1-3 weeks after infection. Intestinal symptoms include abdominal discomfort, severe diarrhea, flatulence, malabsorption and weight loss. In most people, the infection lasts 2-6 weeks, Giardia rarely kills anyone. Boiling water for five to ten minutes or filtering water can prevent infection. Giardia is known to be able to survive chlorine and iodine tablets. There are several prescriptions available for treatment, including Flagyl.
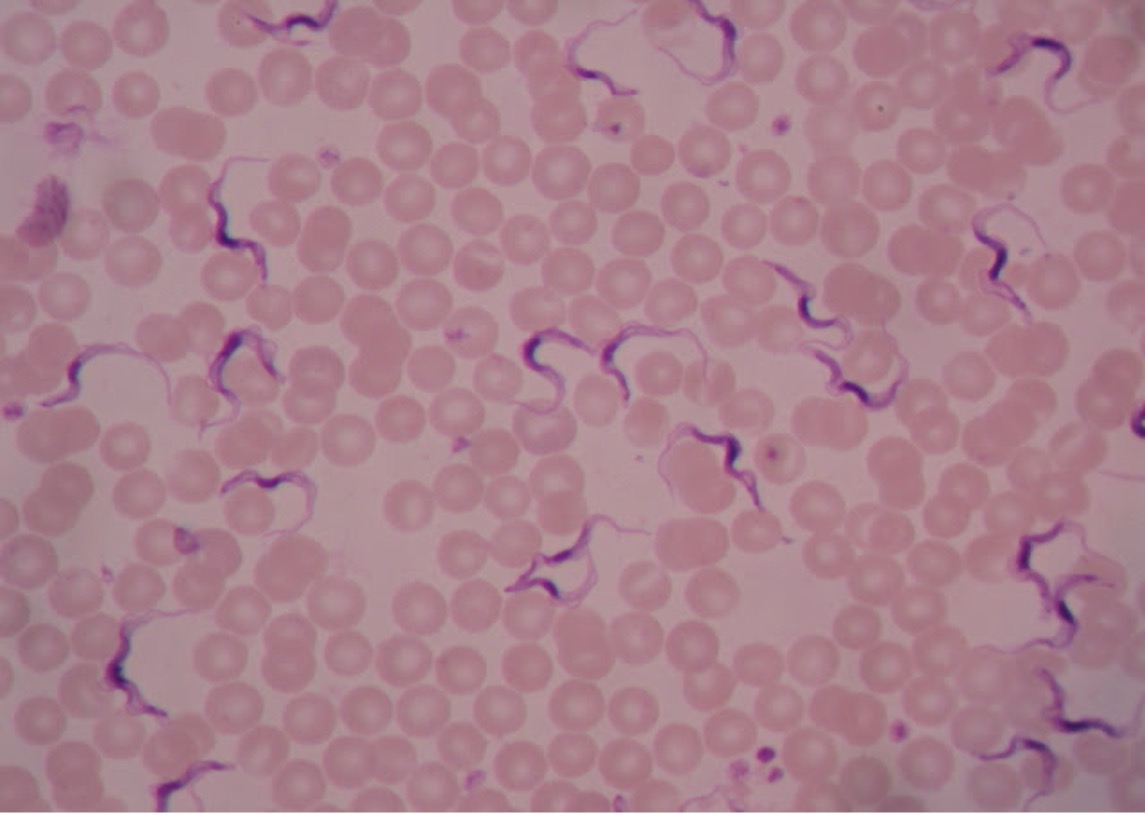
All Trypanosoma appear very similar microscopically. Trypanosomes travel with a wavy spiral motion produced by the contractile flagellum and the undulating membrane. They are visible between the blood cells in blood smears of infected patients. Trypanosomes do not have a cyst stage. Since these parasites are found in the bloodstream, they are called hemoflagellates. Trypanosoma are capable of extreme antigenic variability which stresses the host’s immune system. It appears that Trypanosoma can produce at least one thousand different antigenic coats.
Trypanosoma brucei (trip-an’o-so’muh/bru’see) is the protozoan responsible for causing African sleeping sickness. There are two subspecies of pathogenic Trypanosoma – Trypanosoma brucei gambiense in West Africa and Trypanosoma brucei rhodesiense in East Africa. Both are transmitted by the tsetse fly (Glossina species), which is found only in rural Africa. Although the infection is not found in the United States, historically, it has been a serious public health problem in some regions of sub-Saharan Africa. Since 2015, less than 100 cases have been reported annually to the World Health Organization. Sleeping sickness is curable with Pentamidine in the early stages but is fatal if left untreated.
T. brucei are injected into the host via the bite of an infected tsetse fly. The parasites multiply at the site of inoculation. They then invade the lymph tissue, causing symptoms of fever, headache, nausea, vomiting, and central nervous system involvement leading to coma and death. The tsetse fly infects not only humans, but also cattle, horses, goats, and pigs. Over four million square miles of Africa is unsuitable for raising domestic cattle because of the presence of tsetse flies.
Trypanosoma cruzi (trip-an’o-so’muh/kroo’zye) is the protozoan responsible for causing Chagas disease. T. cruzi is limited to the western hemisphere, including California, the southern United States, Central and South America.It is most often seen in rural areas in Latin America where poverty is widespread. The vector for T. cruzi is the reduviid bug (kissing bug). Humans contract Chagas disease (South American Trypanosomiasis) when an infected bug bites the human skin and subsequently defecates in the wound. Infection may be mild or asymptomatic. There may be fever or swelling around the bug bite. Parasites may also be found in the circulating blood. Chagas disease occurs immediately after infection and may last a few weeks or months. In 20-30% of infected people, acute infection may result in severe inflammation of the heart muscle or the brain and lining around the brain.
Protozoans with Cilia
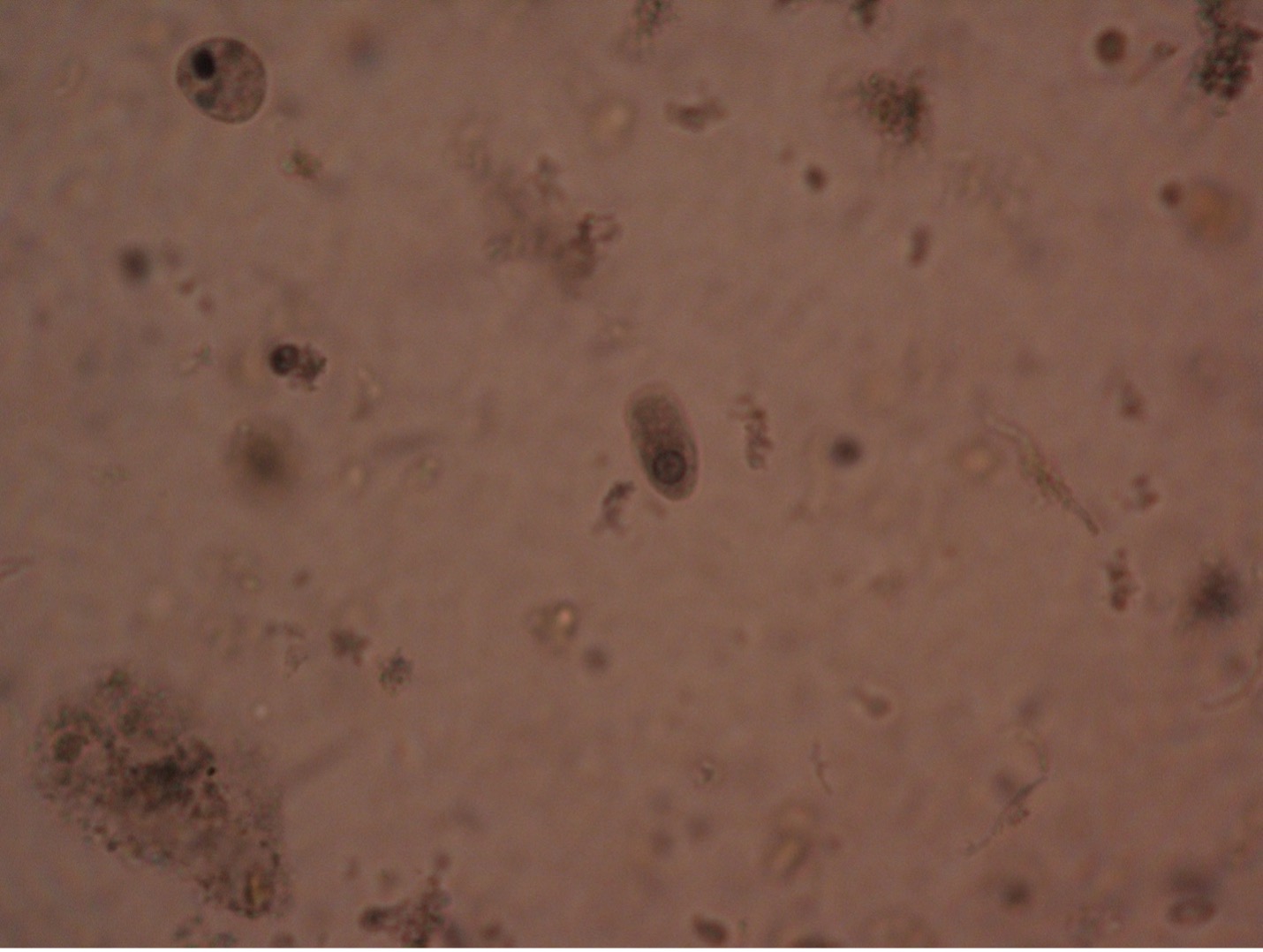
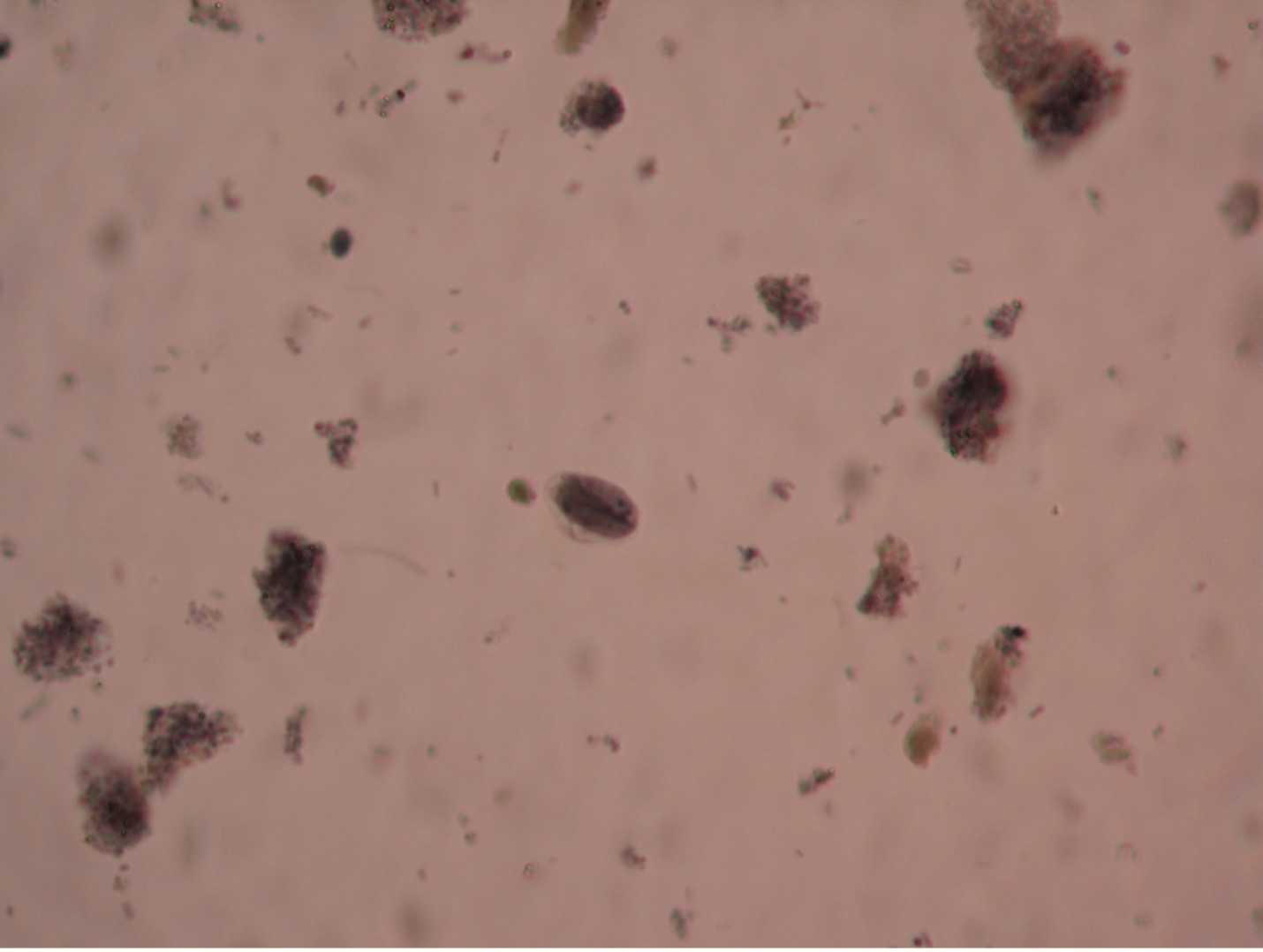
Balantidium coli (bal’-an-tid-ee’um/ko’-lie) is the only known ciliated parasite to infect humans. The parasite is found throughout the world but is rare in the United States. It is most prevalent in tropical and subtropical regions in areas where pigs are raised since they are a common animal reservoir. The parasite is transmitted by the fecal-oral route. The most common clinical manifestations of infection are chronic, recurrent diarrhea alternating with constipation. Balantidium coli dwell in the lumen of the large intestine and may penetrate the intestinal mucosa, causing ulceration. Balantidium is easily recognizable by its large size. The trophozite measures between 50-130 µm long by 20-70 µm wide. It is the largest intestinal protozoan of humans.
Protozoans with no motility
Plasmodium, Cryptosporidium, and Toxoplasma are all genera that are grouped as apicomplexans. Organisms in this group have no means of locomotion. They are all obligate parasites; there are no free-living forms. Their complex life cycles are characterized by alternating asexual and sexual cycles in different hosts. Hosts that harbor the form of the parasite that reproduces sexually are referred to as the definitive hosts. Hosts where asexual reproduction occurs are called intermediate hosts.
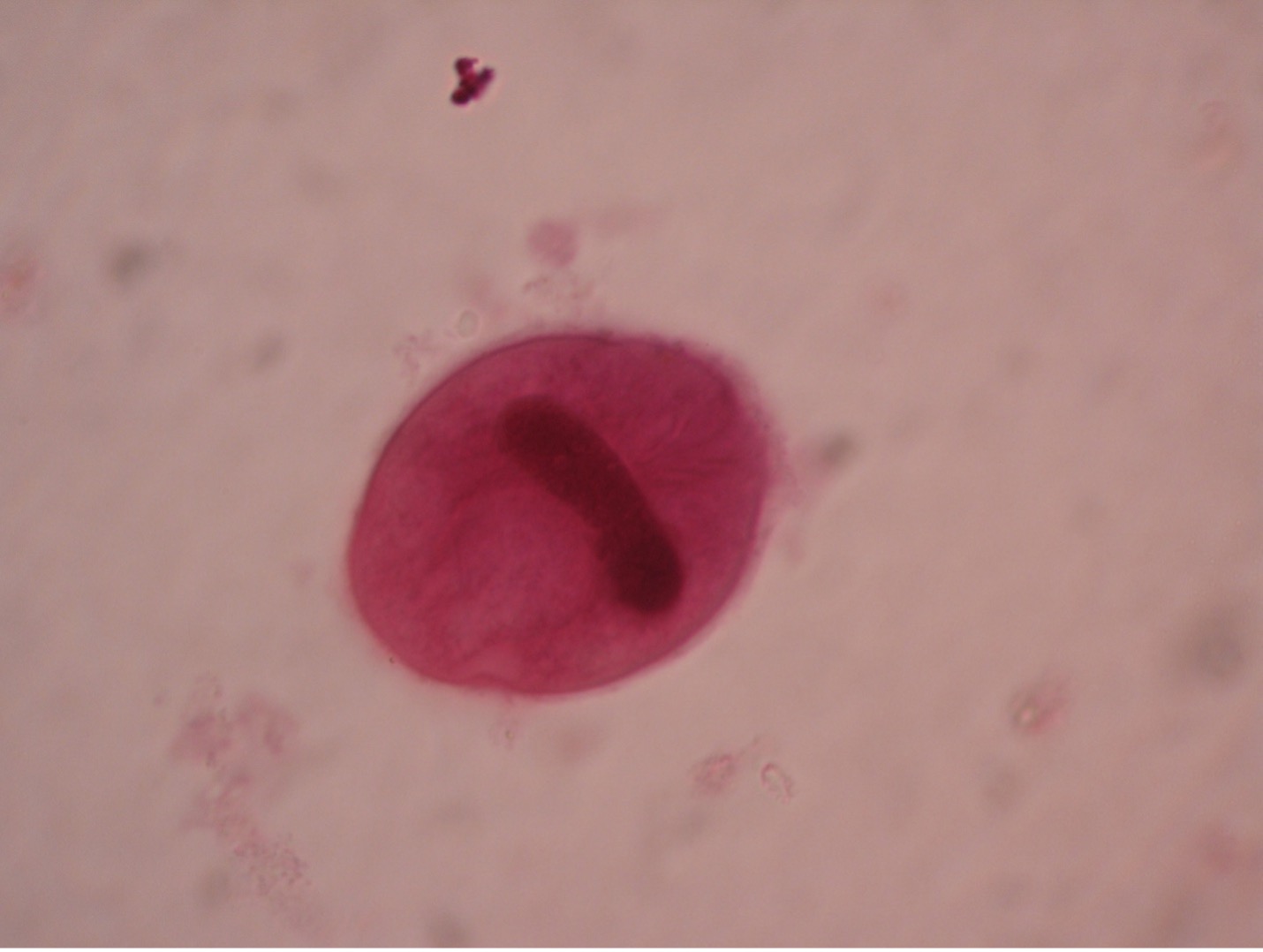
Plasmodium (plaz-mo’dee-um) causes malaria, one of the common causes of hemolytic anemia worldwide. There are four species of Plasmodium – P. vivax (most common), P. ovale, P. malariae, and P. falciparum (most lethal). Typical symptoms are fever, chills, headache, muscle pain, and sweating. According to WHO, there were an estimated 229 million cases of malaria per year worldwide, mostly (94%) in the African Region. Cases of malaria diagnosed in the United States were mostly seen in travelers and immigrants returning to the U.S. from countries where malaria is endemic.
Malaria is the number one cause of death by parasites in the world. To continue existing, it must alternate sexual and asexual cycles. Interruption of either life cycle will control the disease. Measures taken to interrupt the life cycle include attempts to eliminate the Anopheles mosquito, to protect the host from being bitten using chemical repellants and mosquito netting, to prophylactically treating travelers in high risk areas, to cure active cases with various antiparasitic drugs. The occurrence of drug resistant strains worldwide has dramatically increased in recent years. Contact with CDC will aid in determining the best prophylactic drug and drug of choice for treatment depending on the patient’s health and the area in which malaria was acquired.
Plasmodium – Asexual stage in humans
One becomes infected with Plasmodium through the bite of the infected female Anopheles mosquito. The mosquito transmits Plasmodium sporozoites via her saliva when she inserts her proboscis into human skin to obtain a blood meal. The blood provides nourishment for the eggs she will lay. Sporozoites injected into the blood stream leave the blood vascular system within a period of forty minutes and invade the parenchymal cells of the liver. In liver cells, the sporozoites undergo asexual multiplication. They are then liberated and invade red blood cells, initiating the blood stream phase of the infection.
An asexual cycle, known as schizogony, takes place within the red cells of the infected host. This process results in the formation of four to thirty-six new parasites in each red cell. Immature trophozoites, called a “ring” forms, develop which then enlarge to become mature trophozoites, filling most of the parasitized red blood cells. Asexual multiplication occurs when the trophozoites’ nuclear material and cytoplasm split. At the end of the schizogonic cycle the infected blood cells rupture, liberating merozoites, which, in turn, infect new red blood cells. Lysis of the red cells liberates products of metabolism of the parasites and the red cells. These toxic materials cause the symptoms of malaria – chills, fever, nausea, vomiting and headache. The fever spikes occur at varying intervals of 24, 48, or 72 hours depending on the species of Plasmodium present. The febrile period may last several hours, ending with a profuse sweating stage. This cycle is repeated many times.
Plasmodium – Sexual stage in Anopheles mosquito
Sexual stage male and female gametocytes may also appear in the red blood cells. When the mosquito bites an infected person, she draws blood into her stomach which may contain male and female gametocytes. In the mosquito’s gut, the male gametocytes form spermatozoa and the female forms an ovum. Fertilization takes place. The resulting zygote can invade the gut wall and produce numerous sporozoites. The sporozoites migrate through the tissue of the mosquito to the salivary glands where they will be injected into the next human host when the mosquito takes another blood meal. The asexual cycle then proceeds in the new host.
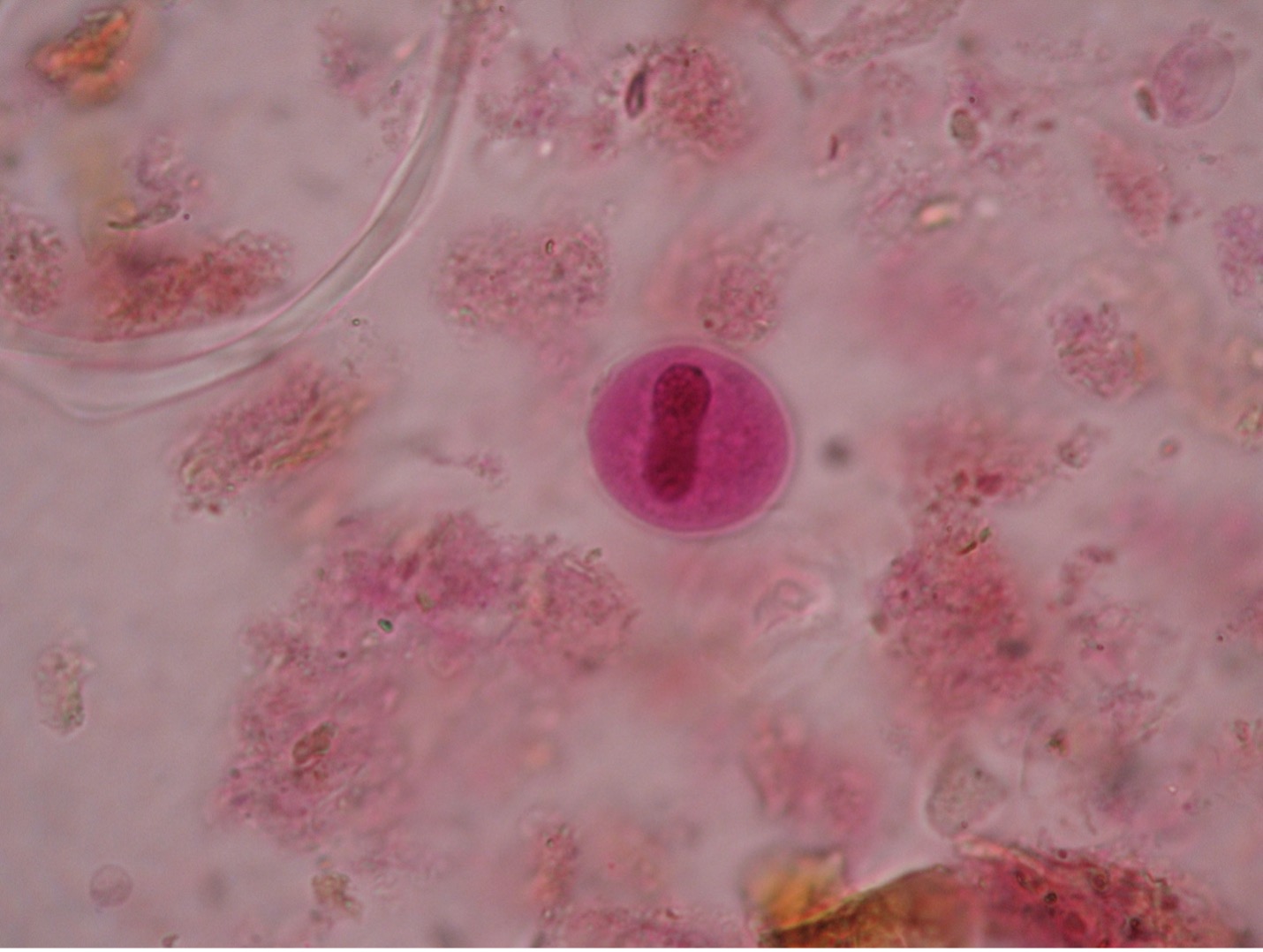
Toxoplasma gondii (tock’so-plaz’muh/gon’dee-eye) is found worldwide and more than 40 million people in the U.S. are infected even though very few have symptoms. A healthy person’s immune system can keep the parasite from causing infections. The people at risk for developing severe infections include pregnant women who can pass the infection to her unborn child and those who are immunocompromised. While the woman is usually asymptomatic, the fetus can develop severe congenital defects such as retinochoroiditis, mental retardation, often due to hydrocephaly or “water on the brain”, convulsions, blindness, and death. T. gondii has been recognized as a prominent opportunist that may cause death in AIDS patients as they suffer the effects of a crippled immune system. While most people do not need treatment, symptomatic human infections occurring in other than pregnant women may be treated with pyrimethamine and sulfadiazine.
The most common way that Toxoplasma is transmitted is through ingestion of the tissue form of the parasite in undercooked, contaminated meat (especially pork, lamb and venison) or accidental ingestion through utensils or handling the raw meat. Hand washing and sufficiently cooking meat before it is ingested are the best prevention.
Another form of transmission may be through infected cat feces. Cats may acquire Toxoplasma by eating an infected mouse or rat. The cat is the definitive host and usually does not exhibit symptoms. The trophozoites of Toxoplasma reproduce sexually in the cat’s intestinal tract. Oocysts, which contain eight sporozoites, are passed in the cat’s feces and may contaminate food or water than can be ingested by other animals. If the oocysts are ingested by humans (or any mammalian or avian animal), the sporozoites will reproduce asexually in the brain, eye, or skeletal muscle tissues of the new host.
It is advisable for pregnant women to avoid all unnecessary contact with cats, especially with their litter boxes. Oocysts require a minimum of 24 hours after passage to sporulate. Thus, one potential method of minimizing exposure to T. gondii sporozoites would be to have the cat’s litter box changed daily. Once the oocysts sporulate and become infective, they may remain infective for up to 18 months in the soil. Litter boxes may be disinfected with boiling water. Children’s sandboxes should have a cover to prevent cats from defecating in them.
Diagnosis of toxoplasmosis often relies on serologic measurement of antibody production. A rising antibody titer would be evidence of current infection. If tissue or fluid suspected of containing parasites is available, it can be inoculated into laboratory mice that are very susceptible to infection. A positive result is indicated by actual isolation of the Toxoplasmafrom the mice or by their development of antibodies to Toxoplasma. Toxoplasma occurs as crescent shaped organisms in body fluids.
Cryptosporidium (krip’toe-spor-i-dee’um), first reported in humans in 1976, has become recognized as one of the most common causes of waterborne disease (recreational water and drinking water) in humans in the United States. The parasite is found in every region of the United States and throughout the world. The parasite can infect every vertebrate family and can readily cross host species barriers. Person to person transmission of Cryptosporidium is likely to happen frequently. Outbreaks of cryptosporidiosis have been reported among children in day-care centers, hospitals, and after waterborne incidents. Outbreaks have occurred following exposure in swimming pools and water parks. Recently the public swimming pools and “splash parks” in the Phoenix area were closed due to Cryptosporidium contaminated water.
A study conducted in the early 1990’s found Cryptosporidium parvum oocysts to be present in 65-95% of water in the United States. There have been large outbreaks from contaminated public water supplies. An outbreak in Maine associated with apple cider was blamed on an infected calf grazing in an area where apples were collected from the ground. A single infected calf can shed up to 10 billion oocysts in one day! The oocysts can remain active for up to 18 months when sequestered in a pile of manure.
Cryptosporidium invades and replicates in the epithelial cells lining the human digestive and respiratory tracts. Rounded oocysts undergo sporogeny within the host cell and are immediately infective when released in the feces. Each mature oocyst contains four sporozoites. Infection is spread by the fecal-oral route – oocysts are transmitted from feces of one infected animal and ingested by another.
Illness due to Cryptosporidium is termed cryptosporidiosis. The most common symptom is watery diarrhea, but other symptoms include stomach cramps, dehydration, nausea and vomiting. It is thought to be a significant cause of morbidity and mortality, especially in young children living in developing countries. In people with healthy immune systems, the diarrhea lasts three to twelve days, yet the cysts can be shed for 2 weeks after the symptoms subside. In immunodeficient persons, such as AIDS patients, life-threatening, cholera-like illness may occur. Cryptosporidiosis is one of the more important opportunistic infections of AIDS patients.
The thick wall of the oocyst makes Cryptosporidium extremely resistant to most common disinfectants. They can survive in undiluted bleach for hours and will survive standard chlorination of drinking water. The most effective way to prevent infection is to subject water to microfiltration using filters with pores no larger than 0.2 micrometers.
Diagnosis is achieved through finding oocysts in stool specimens from infected patients. The oocysts are difficult to see with routine stains. A modified acid-fast stain may be used in which stained oocysts appear bright red against a blue-green background. Sometimes the four sporozoites may be seen within the oocysts wall. Although most healthy people will recover without treatment, nitazoxanide is the drug of choice to treat cryptosporidiosis.
PRE-ASSESSMENT
procedure
Draw microscope demonstrations on the worksheet.
PARASITE PHOTOS
POST TEST
DISCOVERIES IN MICROBIOLOGY
DR. BRUCE AMES
 Do you prefer to buy products not tested on animals? The Ames test was developed by American biochemist Dr. Bruce Ames in the 1970’s to determine if a chemical is a mutagen. The Ames test uses a mutant strain of Salmonella that cannot produce the amino acid histidine. If the chemical reverses the mutation, it is a mutagen of Salmonella. The chemicals that are mutagenic by the Ames test may be further tested on animals to assess their ability to cause mutations/cancer in humans.
Do you prefer to buy products not tested on animals? The Ames test was developed by American biochemist Dr. Bruce Ames in the 1970’s to determine if a chemical is a mutagen. The Ames test uses a mutant strain of Salmonella that cannot produce the amino acid histidine. If the chemical reverses the mutation, it is a mutagen of Salmonella. The chemicals that are mutagenic by the Ames test may be further tested on animals to assess their ability to cause mutations/cancer in humans.

