12 NEGATIVE STAIN
LEARNING OBJECTIVES
Perform the negative staining technique
Identify the presence of bacterial capsules
Explain why bacterial capsules do not stain with traditional stains
Discuss how bacterial capsules benefit the bacteria that produce them
MCCCD OFFICIAL COURSE COMPETENCIES
Utilize aseptic technique for safe handling of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cell
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
MATERIALS
Stock Culture:
Klebsiella aerogenes in Litmus Milk
Equipment:
2 glass microscope slides
2 coverslips
Dental floss
Toothpick
Inoculating loop
Test tube rack
Microscope
Stains:
Anthony’s capsule stain:
1% solution of crystal violet
20% solution of copper sulfate
Nigrosin or India ink
Most staining procedures in the microbiology laboratory (simple, Gram, endospore, and acid fast stains) use a positive stain technique. In a positive stain the microorganisms are stained and the background is not stained. Many bacteria secrete a rather sticky substance that adheres to the cell and forms a coating around it called a glycocalyx. If this structure is very loosely bound and somewhat irregularly shaped, it is called a slime layer. The slime layer may not be easy to see. If the structure is quite tightly bound, highly organized, and generally round or oval, it is called a capsule. Capsules are generally easier to visualize than slime layers. Capsules may be made of polysaccharides, glycoprotein, or polypeptides. The capsule aids the bacteria in invading the human body by helping it to resist phagocytosis by white blood cells. The capsule is sticky and helps bacteria attach to skin and mucous membranes. It is a source of nutrition, and some bacteria may find the need to metabolize their capsules as an energy source if food in their environment runs out. The capsule contributes to the virulence of various microbes. Streptococcus pneumoniae, for example, can be found as part of the normal microbiome of mucous membranes, yet it is pathogenic when it produces a capsule.
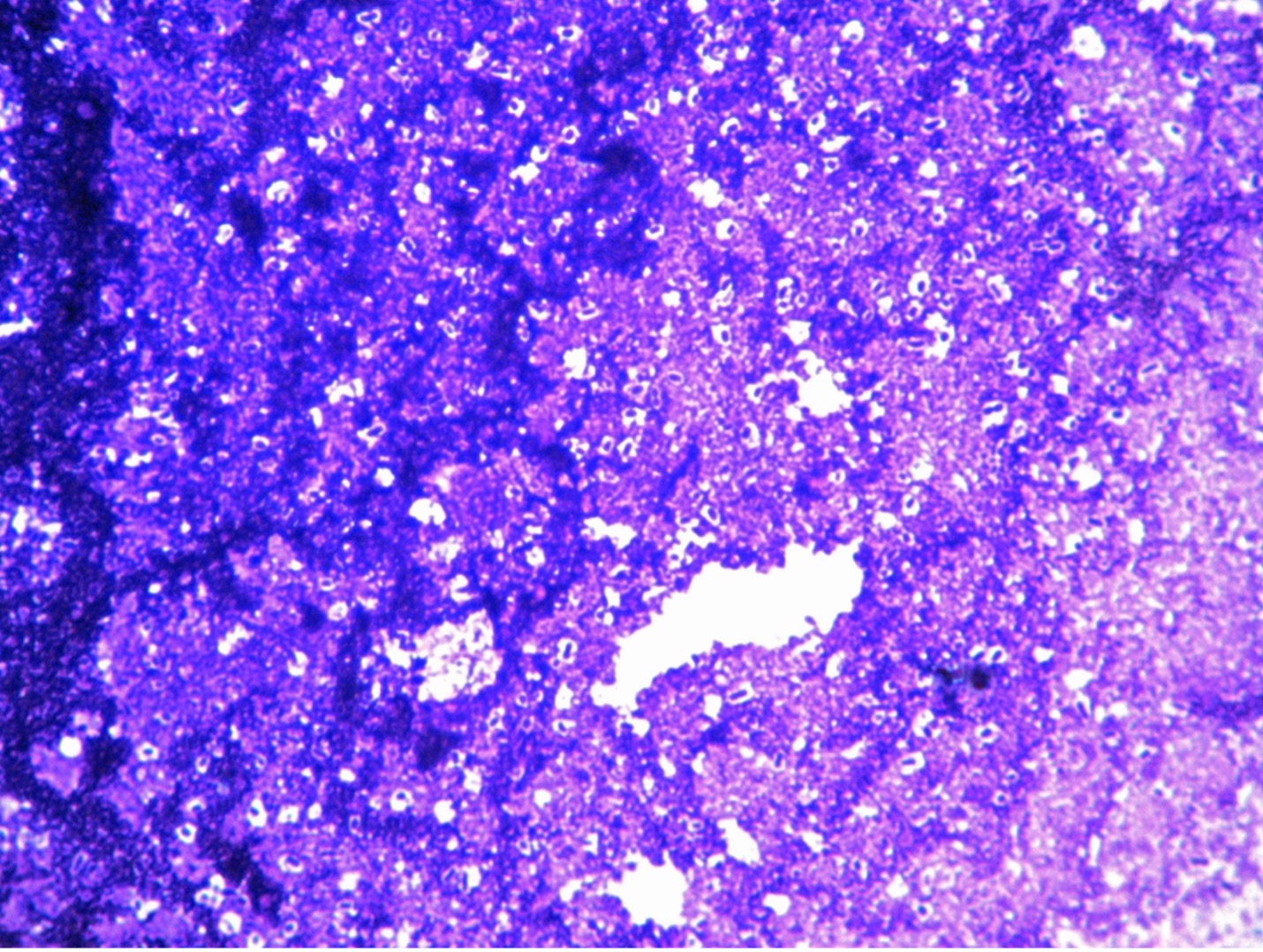
Capsules are non-ionic (no net charge) meaning capsules will not stain with a positively charged stain or a negatively charged stain. We need to utilize negative staining techniques to view the outline of the capsule. Negative stains are repelled by the negatively charged bacterial cells preventing the cells from taking up the stain. Therefore, in negative staining the background (rather than the microorganism) is stained. Nigrosin and India ink are both examples of negative stains commonly used in microbiology, although any negatively charged stain maybe used. Heat damages certain cellular features including bacterial glycocalyx (capsules and slime layers), therefore we do not heat fix when negative staining. Bacterial capsules are soluble in water, so we do not rinse with water in capsule staining. The Anthony’s method for staining capsules uses litmus milk to provide a proteinaceous background for contrast. Since the capsules do NOT pick up any stain, the results produce a blue background, clear outline of the capsules, and dark blue microorganisms inside the capsule.
PRE-ASSESSMENT
PROCEDURE
Prepare a bacterial smear for Capsule Stain (Anthony Method
For this exercise – Use Klebsiella aerogenes in litmus milk broth
1. Obtain a clean glass slide.
2. Sterilize an inoculating loop and allow it to cool.
3. Remove the cap of the Klebsiella in the litmus milk, insert the inoculating loop and obtain a loopful of inoculum. Gently place the inoculum on the slide.
4. Sterilize the loop, allow it to cool and add another loopful of inoculum onto the slide. The smear will be very thick.
5. Allow the smear to thoroughly dry. DO NOT HEAT FIX. Heat fixing will shrink the organisms and either produce artifacts that look like capsules or the heat will destroy the capsules that are present.
6. Add 1% crystal violet to the smear. Allow the dye to remain on the slide for 2 minutes.
7. Rinse the slide with copper sulfate. DO NOT RINSE WITH WATER so you do not dissolve the capsules that are present.
8. Shake the copper sulfate from the slide. Lightly blot off excess solution with bibulous paper or paper towel. The smear is VERY fragile, and it is easy to blot the smear off the slide.
9. Place a drop of immersion oil on the smear and then place a cover slip on top. Then add an oil drop to the top of the cover slip.
10. View the smear using the oil immersion lens of the microscope. Move the smear to a thin layer of the inoculum to view the capsules properly.
11. Draw your observations on the worksheet. Label the capsule and the bacterial cell.
OPTIONAL EXERCISE:
Negative Stain of Organisms in the Mouth
1. Obtain a toothpick, two glass slides and nigrosin stain.
2. Place a very small drop of nigrosin near one end of one of the glass slide.
3. Use a toothpick to scrape in between your teeth to obtain plaque.
4. Smear the plaque from the toothpick in the drop of nigrosin on the slide.
5. Use the second clean glass slide as a “spreader”. Touch the edge of the nigrosin drop with the edge of the spreader slide at a 45-degree angle.
6. After the nigrosin spreads along the edge of the spreader slide, quickly push the slide to spread out the drop on the bottom slide. Discard the spreader slide in disinfectant.
7. Allow the slide to completely air dry.
8. Observe several fields until you see clear-white bacterial shapes in a dark background.
9. Draw your observations in the worksheet.
POST TEST
DISCOVERIES IN MICROBIOLOGY
 DR. LOUIS PASTEUR
DR. LOUIS PASTEUR
During the French Revolutionary war, the French army offered $12,000 dollars to anyone who could develop a way to safely store food. After fourteen years of experimentation, French candy maker Nicolas Appert developed the canning process. Food is placed in jars or cans and and heated to a temperature that kills microorganisms. As the jars or cans cool, a vacuum seal is formed which prevents other microorganisms from getting in. in1863, Emperor Napoleon III recruited French chemist and microbiologist Dr. Pasteur to save France’s wine industry from the “diseases of wine”. In previous experiments, Pasteur had discovered that heating the fermented wine would kill the microorganisms that caused it to spoil. Pasteur determined the exact time and temperature that would reduce spoilage microorganisms and kill pathogenic microorganisms without changing taste. He patented the process and called it pasteurization. Before long, the process was used for many beverages and foods.

