22 MIXED UNKNOWNS
LEARNING OBJECTIVES
Demonstrate competency of microbiology laboratory techniques by separating and identifying two organisms in a mixed broth
Design two dichotomous keys to differentiate the possible Gram-negative unknowns and the possible Gram-positive unknowns
MCCCD OFFICIAL COURSE COMPETENCIES
Describe the modes of bacterial and viral reproduction and proliferation
Utilize aseptic technique for safe handling of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cell
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
MATERIALS
Unknown mixed broth containing Gram-positive cocci and Gram-negative bacilli
1 MacConkey Agar
1 CNA Agar
Inoculating loop
Sterile swab
BIOCHEMICAL TESTS ALBUM LINK
| Possible Gram Positive Unknowns: | Possible Gram Negative Unknowns: | |
| Staphylococcus aureus | Pseudomonas aeruginosa | |
| Staphylococcus epidermidis | Alcaligenes faecalis | |
| Micrococcus luteus | Escherichia coli | |
| Micrococcus roseus | Klebsiella aerogenes | |
| Group C Streptococcus | Proteus mirabilis | |
| Streptococcus pyogenes | Klebsiella pneumoniae | |
| Streptococcus pneumoniae | Salmonella enterica | |
| Streptococcus sanguinis | Shigella flexneri | |
| Enterococcus faecalis |
There are two different types of bacteria in the broth of the mixed unknown. One type is Gram-positive cocci and the other is Gram-negative bacilli. First, you must isolate and separate the two organisms from each other by using selective media.
The MacConkey Agar is selective for Gram-negative organisms. The crystal violet dye and the bile salts in the media inhibit the growth of Gram-positive organisms. MacConkey agar is also a differential media because it contains lactose. Gram-negative bacilli that ferment lactose will appear hot pink and those that do not ferment lactose will appear colorless.
The Colistin-Nalidixic Agar is selective for Gram-positive organisms. CNA contains the antibiotics colistin and nalidixic acid which suppress the growth of Gram-negative organisms. CNA is differential because it contains red blood cells. You can determine the hemolytic properties of the Gram-positive cocci.
Once the bacteria have been isolated from each other, the next step is to identify both bacteria. By using biochemical test charts to differentiate the Gram-positive cocci and the Gram-negative bacilli, you will design two dichotomous keys (flow charts) to guide you in determining which tests will help you identify your unknowns. The result of the first test will determine the next test to perform.
You must complete the tests in the order of the dichotomous key until you reach an identification of your unknowns. Record the biochemical tests you perform in the order of your dichotomous key. Record the identification of the unknown using correct binomial nomenclature.
PRE-ASSESSMENT
PROCEDURE
Day 1: Inoculate selective/differential media
1. Label the MacConkey and CNA plates with your name (not initials), date, unknown number and the name of the media. Record your unknown number on the worksheet.
2. Inoculate your unknown broth onto the MAC and the CNA media. To ensure enough sample is collected, use a sterile cotton-tip swab to obtain the inoculum. Roll the swab onto the first section ONLY. Then, use a sterile inoculating loop to continue streaking for isolation. Use your best aseptic technique and streak for isolation.
3. Stab the CNA plate in the first section to enhance the hemolysis.
4. Incubate your MAC plate upside down in the class plate tray until the next lab period.
5. Incubate your CNA plate upside down in a candle jar since some of the unknown Gram-Positive organisms are microaerophilic.
6. Label your mixed unknown broth with your name and lab section (day & time). Place the broth in the rack on the front table of the lab. These tubes will be saved in the refrigerator in case your unknowns do not grow on the media.
Day 2: Make Stock Cultures
1. Observe your MacConkey Agar plate and determine if your organism ferments lactose (or not) and record your results on the laboratory report form that you will submit for grading. If you do not have growth, reincubate your plates and subculture the original broth again.
2. Take an ISOLATED colony from the MacConkey Agar and streak for isolation on a TSA plate. Label this TSA with your name, your unknown number and the words “GRAM NEGATIVE”. THIS WILL BE YOUR STOCK CULTURE.
3. You will use this stock culture to inoculate all future biochemical tests performed on this unknown. T. soy agar is a “non-inhibitory” media which allows the organism to produce enzymes and metabolize the ingredients in the media.
4. Incubate the TSA plate of your Gram-Negative stock culture upside down in the class plate tray until the next lab period.
5. Observe an isolated colony growing on your CNA plate. Evaluate the hemolytic properties to determine if the organism produces beta, alpha or gamma hemolysis. (Descriptions of hemolysis are in Ex. 18) Record your observations on the worksheet.
6. Take an ISOLATED colony from the CNA plate and streak for isolation on a TSA plate. Label this TSA plate with your name, your unknown number and the words “GRAM POSITIVE”. THIS WILL BE YOUR STOCK CULTURE. You will use this stock culture to inoculate all future biochemical tests performed on this unknown.
7. Incubate the TSA plate of your Gram-Positive stock culture upside down in the Candle Jar.
8. Put the CNA and MAC plate back in the class tray to be saved in the refrigerator in case your stock cultures do not grow on the TSA plates.
Day 3: Begin testing your unknown organisms
1. As soon as you have your dichotomous keys (flow charts) graded and your organisms growing on stock cultures, you can begin to perform biochemical tests. YOU MUST PERFORM THE TESTS IN THE ORDER ON YOUR FLOW CHART.
2. Use your stock culture to perform the first test listed on your dichotomous key. Label the test media with your name, test name, date, and whether the organism is your Gram Negative or Gram-positive unknown. Incubate the test until the next lab session.
* You can only inoculate ONE test/unknown/day unless you can determine the results of the biochemical test right away.
3. Work on identifying both unknowns simultaneously. Organize your work so you do not mix up the tests. In your test tube rack, place the stock culture you are using in front of the biochemical test you are going to inoculate. Immediately label the test media with your name, date, media name and whether this is the Gram Positive or Gram-negative unknown.
4. Once you have recorded the reactions and results, properly dispose of the test. Do not keep the biochemical tests after you have recorded the results.
5. Once you have identified both unknowns, turn in your worksheets and flow charts. After confirming your results with your instructor, dispose of all your test media, agar plates and broths.
BIOCHEMICAL TESTS RESULTS OF GRAM POSITIVE COCCI UNKNOWN
BIOCHEMICAL TEST RESULTS OF STAPHYLOCOCCUS AND MICROCOCCUS
| TEST | Staphylococcus aureus | Staphylococcus epidermidis | Micrococcus roseus | Micrococcus luteus |
|---|---|---|---|---|
| Catalase | Positive | Positive | Positive | Positive |
| Glucose Fermentation | Positive | Positive | Negative | Negative |
| Coagulase | Positive | Negative | Negative | Negative |
| Salt Tolerance on Mannitol Salt Tolerance | Positive | Positive | Negative | Negative |
| Mannitol Fermentation on Mannitol salt Agar | Positive | Negative | Negative | Negative |
| Pigment Production (colony color) | White | White | Rose-Coral | Yellow |
| Nitrate Reduction (not done in this exercise) | Positive | Positive | Positive | Negative |
BIOCHEMICAL TEST RESULTS OF ALPHA, BETA, AND GAMMA HEMOLYTIC STREPTOCOCCUS AND ENTEROCOCCUS
| TEST | Streptococcus sanguinis | Streptococcus pneumoniae | Streptococcus pyogenes | Group C Streptococcus |
Enterococcus faecalis |
| Catalase | Negative | Negative | Negative | Negative | Negative |
| Bile Esculin | Negative | Negative | Negative | Negative | Positive |
| Hemolysis | Alpha | Alpha | Beta | Beta | *Variable |
| Optochin sensitivity | Resistant (Negative) |
Sensitive (Positive) |
|||
| Latex Agglutination | Positive reaction with Group A antibodies | Positive reaction with Group C antibodies |
NOTE: “Variable” test result means the result is not consistent. Some strains of the species will exhibit gamma hemolysis whereas other strains will produce alpha or beta hemolysis.
BIOCHEMICAL TEST RESULTS OF GRAM NEGATIVE Bacilli UNKNOWN
BIOCHEMICAL TESTS RESULTS OF GRAM NEGATIVE UNKNOWN
| TEST | Escherichia coli | Klebsiella aerogenes |
Klebsiella pneumoniae |
Proteus mirabilis | Salmonella | Shigella | Pseudomonas aeruginosa | Alcaligenes faecalis |
| Oxidase | Negative | Negative | Negative | Negative | Negative | Negative | Positive | Positive |
| Glucose fermentation | Positive | Positive | Positive | Positive | Positive | Positive | Negative | Negative |
| Lactose fermentation | Positive | Positive | Positive | Negative | Negative | Negative | Negative | Negative |
| Sucrose fermentation | Negative | Positive | Positive | Negative | Negative | Negative | Negative | Negative |
| Nitrate reduction | Positive
(NO3->NO2) |
Positive
(NO3->NO2) |
Positive
(NO3->NO2) |
Positive
(NO3->NO2) |
Positive
(NO3->NO2) |
Positive
(NO3->NO2) |
Positive
(NO3->N2 gas) |
Negative |
| Urease | Negative | Negative | Positive | Positive | Negative | Negative | Not reliable | Negative |
| Indole | Positive | Negative | Negative | Negative | Negative | Positive | Not reliable | Not reliable |
| MR Test | Positive | Negative | Negative | Positive | Positive | Positive | Negative | Negative |
| VP Test | Negative | Positive | Positive | Negative | Negative | Negative | Negative | Negative |
| Citrate | Negative | Positive | Positive | Positive | Positive | Negative | Positive | Not reliable |
| KIA H2S production | Negative | Negative | Negative | Positive | Positive | Negative | Negative | Negative |
| Motility | Positive | Positive | Negative | Positive | Positive | Negative | Not reliable | Negative |
DISCOVERIES IN MICROBIOLOGY
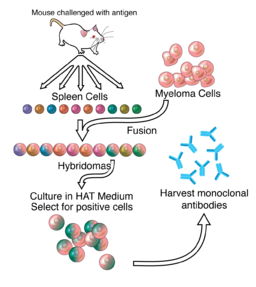 DR. CÉSAR MILSTEIN
DR. CÉSAR MILSTEIN
In 1975, Argentine biochemist Dr. César Milstein was the first to produce monoclonal antibodies. Prior to the development of Dr. Milstein’s technique, scientists had struggled to produce large amounts of single antibody clones because of the difficulty of growing antibody producing B cells in culture. Dr. Milstein overcame this problem by fusing spleen cells derived from mice immunized with a specific antigen to immortalized myeloma cells, a technique that enabled production of antigen-specific antibody in culture indefinitely. His techniques were used to produce diagnostic tests, cancer treatments, disease treatment, vaccines, and blood and tissue typing.

