IDENTIFICATION OF ENTEROBACTERIACEAE
LEARNING OBJECTIVES
Perform and interpret the Indole, MR-VP, Citrate, Urease, Motility and Sucrose fermentation tests
State the purpose and principle of the Indole, MR-VP, Citrate, Urease, Motility and Sucrose fermentation tests
Identify a member of the Enterobacteriaceae by results of these tests
MCCCD OFFICIAL COURSE COMPETENCIES
Describe the modes of bacterial and viral reproduction and proliferation
Utilize aseptic technique for safe handling of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cell
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
MATERIALS
Media: per person
Sucrose fermentation broth
Tryptone Broth
MR-VP Broth
Citrate Slant
Urea Slant
Motility agar
Equipment:
Inoculating wire and loop
Stock Cultures:
Klebsiella aerogenes on TSA slant 8 / lab section at Pecos 6 / lab section at Williams
Escherichia coli on TSA slant TSA slant 8 / lab section at Pecos 6 / lab section at Williams
Proteus mirabilis on TSA slant TSA slant 8 / lab section at Pecos 6 / lab section at Williams
Klebsiella pneumoniae on TSA slant TSA slant 8 / lab section at Pecos 6 / lab section at Williams
Reagents:
Kovac’s reagent
Methyl Red
Barritt’s A and B
BIOCHEMICAL TESTS ALBUM LINK
Gram‑negative bacilli in the family Enterobacteriaceae are the most frequently encountered microbes in the clinical microbiology laboratory. They are often referred to as “enterics” since many of them are part of the normal microbiome of the intestinal tract.
Since most enterics have the same gram stain results and cellular morphology, neither Gram stains nor colonial morphology will aid the microbiologist in identification of these bacteria. Thus, we must perform biochemical tests to detect the presence or absence of metabolic enzymes. These enzymes are encoded in the genetic material of the bacterial genome and thus form a biochemical “fingerprint” that can be used to identify a species. Substrates upon which these enzymes act are incorporated into the culture medium along with an indicator system to allow detection of specific metabolic products.
Historically, the IMViC tests were one of the earliest tests developed for identifying enterics. IMViC is an acronym that stands for indole, methyl red, Voges‑Proskauer, and citrate tests. (The lowercase letter i is included for ease of speech.) The presence of Escherichia coli was used by public health officials for many years to indicate fecal contamination of food and water supplies. While Enterobacter resembles E. coli because they both ferment lactose, the presence of Enterobacter was not thought to necessarily indicate fecal contamination because it is widespread in soil and grass.
Escherichia coli, Klebsiella, Enterobacter and Proteus are often part of the normal intestinal microbiome and are non‑pathogens in that environment. Of course, these bacteria can cause illness under other circumstances. For example, E. coli is a common cause of urinary tract infections and Proteus has been isolated from patients with kidney stones, gangrenous wounds, and otitis media.
The truly enteric pathogens in the Enterobacteriaceae family include Salmonella, which causes typhoid fever and mild to severe gastroenteritis (inflammation of the gastrointestinal tract) due to “food poisoning” and Shigella, which causes bacterial dysentery (bloody diarrhea with fever) due to “food poisoning”.
Many commercial kit systems are available to identify Enterobacteriaceae. Two of these commercial systems are the Enterotube and the API20E. Even though these systems are miniaturized and compact, they still test for bacterial enzymes to identify the bacteria.
PRE-ASSESSMENT
PROCEDURE
For this exercise: Work in groups of 3 at Pecos groups of 4 at Williams. Each person in the group will work with one of the color dot cultures.
SUCROSE broth FERMENTATION TEST
The sucrose fermentation broth includes the sugar sucrose and a pH indicator to determine if bacteria can ferment sucrose into acidic end products.
1. Obtain the sucrose fermentation broth.
2. Use a permanent marker to write the color dot, your name (or initials), and the name of the media (sucrose) on the side of the tube.
3. Using a sterile inoculating loop, obtain a small amount of bacteria and inoculate the tube.
4. Incubate the fermentation tubes in the class test tube rack until the next lab session.
AFTER INCUBATION–Observe the appearance of the sucrose fermentation tube. Record the result on the worksheet. Dispose of the sucrose fermentation tubes in the discard rack.
Yellow broth = Positive = ferments sucrose
No color change (stays red) = Negative

UREASE TEST
This test detects the ability of the bacteria to produce the enzyme urease. The media contains urea and the pH indicator phenol red. Urease will hydrolyze urea to produce ammonia. The alkaline environment will change the phenol red indicator to hot pink.
1. Obtain a urea agar slant. Use a permanent marker to write the color dot, your name (or initials), and the name of the media (urea) on the side of the tube.
2. Using a sterile inoculating loop, obtain a heavy amount of bacteria and streak the entire slant of the Urea agar.
3. Incubate the inoculated tube in the class test tube rack until next lab session.
AFTER INCUBATION-Observe the urea media for a color change. Record the result on the worksheet. Dispose of the urea slants in the discard rack.
Hot pink media= Positive
No color change (stays light salmon) = Negative
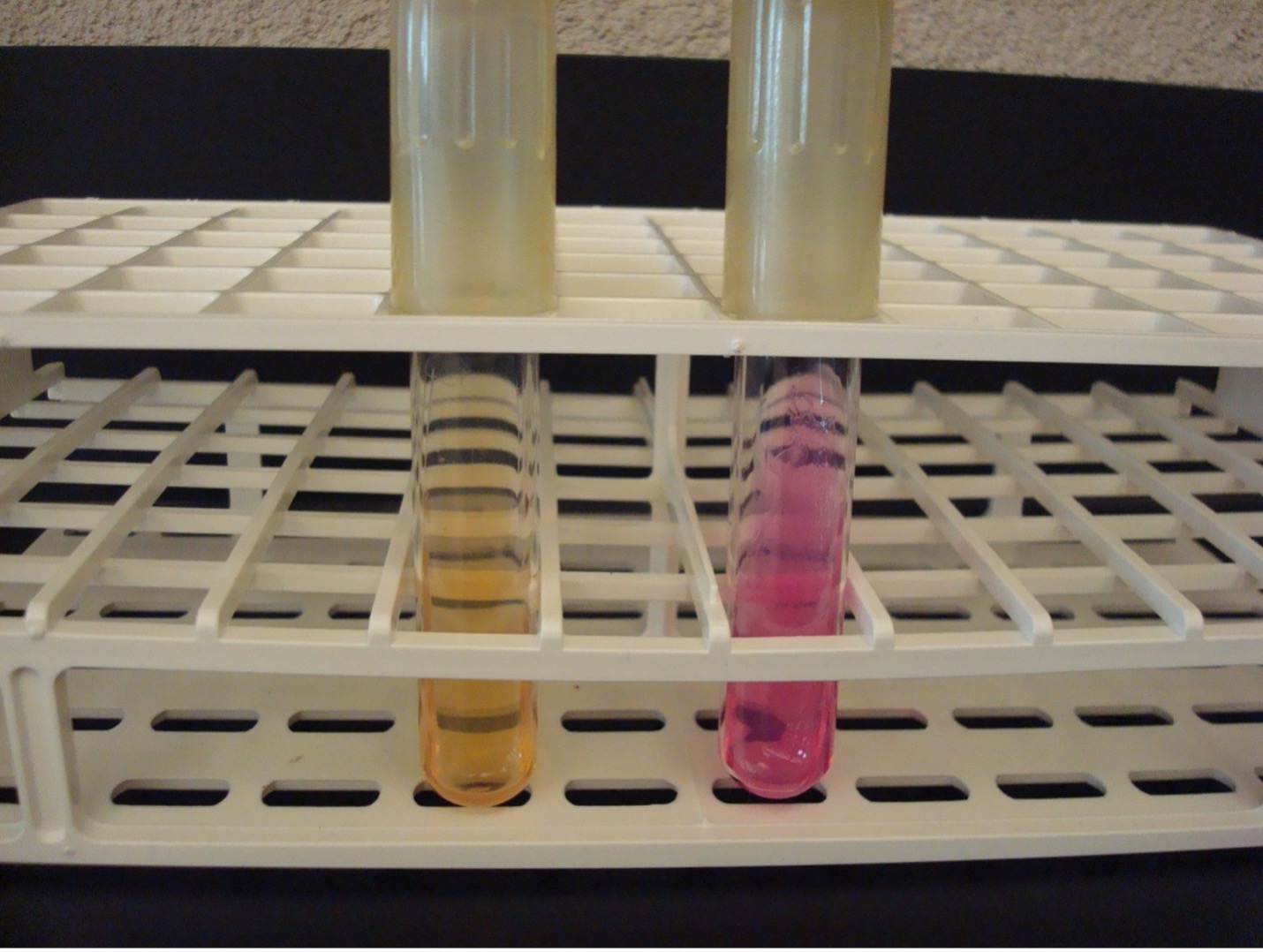
INDOLE TEST using tryptone broth
The Indole test uses tryptone broth to detect the organism’s ability to produce tryptophanase. This enzyme will split the amino acid tryptophan into indole and pyruvic acid. Indole forms a red ring with the addition of Kovac’s reagent.
1. Obtain a tryptone broth. Use a permanent marker to write the color dot, your name (or initials), and the name of the media (tryptone) on the side of the tube.
2. Using a sterile inoculating loop, obtain a small amount of bacteria and inoculate the culture into the Tryptone broth.
3. Incubate the inoculated tube in the class test tube rack until next lab session.
AFTER INCUBATION-Add 10 drops of Kovac’s reagent to the tryptone tube. Record the reaction/results on the worksheet. Kovac’s reagent has an unpleasant odor. Dispose of the tryptone tubes in the discard rack that will be removed from the lab when lab is over.
Red ring = Positive
No red ring = Negative
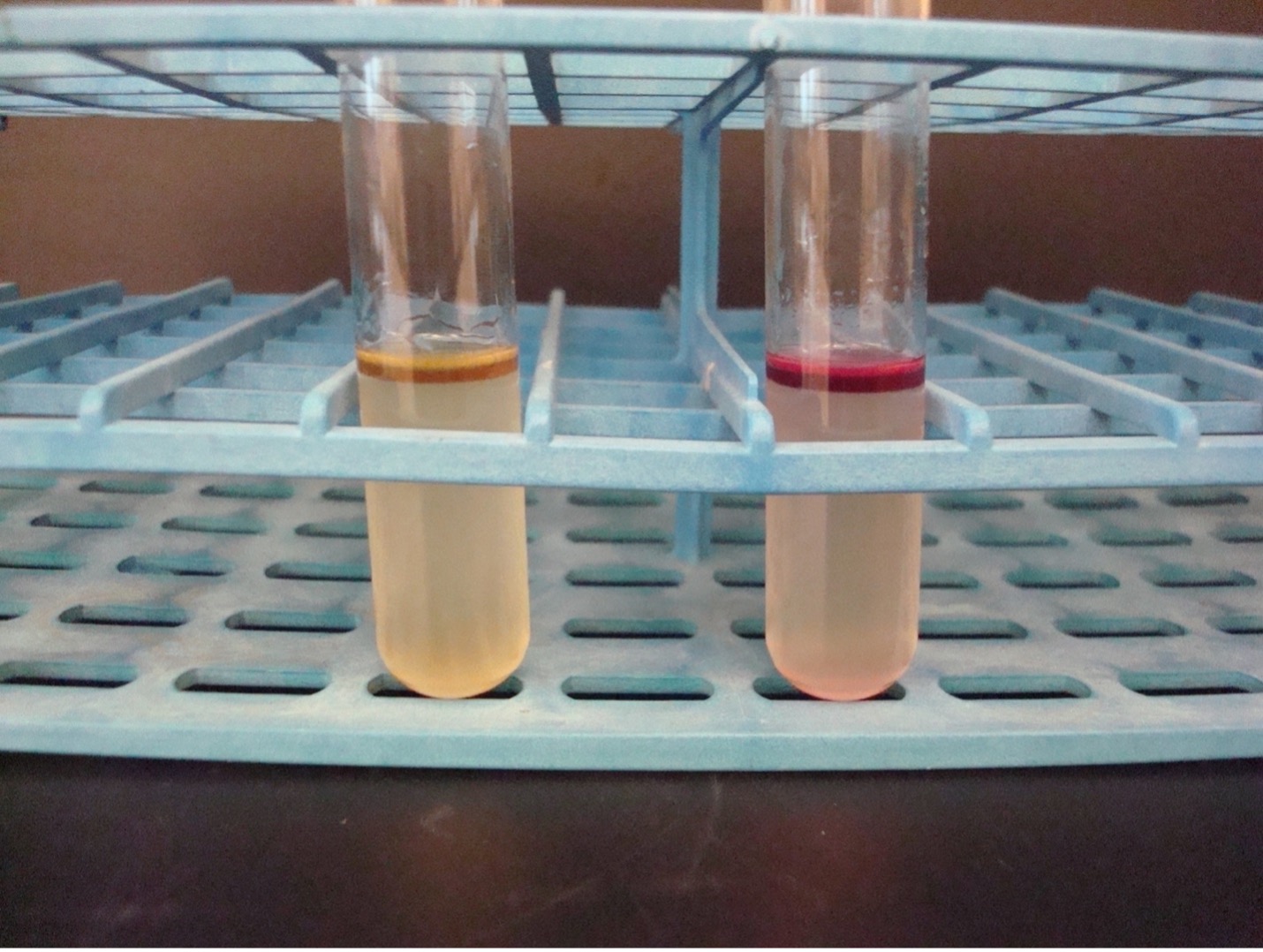
METHYL RED–VOGES PROSKAUER broth TEST
The MR-VP test is two separate tests that uses one broth of glucose to determine the end product of glucose fermentation. Some bacteria ferment glucose using mixed acid fermentation pathway (detected in the MR test) while other bacteria ferment glucose via the butanediol fermentation pathway (detected in the VP test).
MRVP Broth (Glucose) —–> Pyruvic acid ——> Mixed acid fermentation pathway (MR+)
OR
MRVP Broth (Glucose) —–> Pyruvic acid ——> Butanediol fermentation pathway (VP+)
1. Obtain a MR-VP broth tube. Use a permanent marker to write the color dot, your name (or initials), and the name of the media (MR-VP) on the side of the tube.
2. Using a sterile inoculating loop, obtain a small amount of bacteria and inoculate the MR-VP broth.
3. Incubate the inoculated tube in the class test tube rack until next lab session.
AFTER INCUBATION
1. Use a sterile transfer pipette to transfer half of the inoculated MR-VP broth to a clean test tube. Label this tube “VP”. Leave the other half of the inoculated broth in the original MR-VP broth test tube. Label this tube “MR”.
2. MR TEST Add 10 drops of Methyl Red reagent to “MR” tube. Methyl red is a pH indicator that will detect mixed acid production. Record the reactions and results on the worksheet. Methyl red has an unpleasant odor. Dispose of the MR tubes in the discard rack that will be removed from the lab when lab is over.
3. VP TEST First add 15 drops of Barritt’s A reagent (alpha-napththol) and then add 5 drops of Barritt’s B (40% KOH) to the “VP” tube. NOTE: A reversal in the order of the reagents may result in a weak-positive or false-negative reaction. Mix the tube well by flicking it with your fingers and then let it sit undisturbed in a test tube rack for 20 minutes. A positive reaction indicates the bacteria ferment glucose via the butanediol fermentation pathway. Record the reactions and results on the worksheet. Barritt’s A and B have an unpleasant odor. Dispose of the VP tubes in the discard rack that will be removed from the lab when lab is over.
Red broth = MR Positive
Yellow broth = MR Negative

Dark red-brown color at the top of the tube = VP Positive
Yellow color at the top of the tube = VP Negative

CITRATE TEST
This test uses citrate agar to determine if the organism can use citrate as its only carbon source. Since all organisms need carbon to survive, bacteria that cannot use the citrate will not grow. The agar contains citrate and ammonium ions (nitrogen source) and bromthymol blue as a pH indicator.
1. Obtain a Citrate tube. Use a permanent marker to write the color dot, your name (or initials), and the name of the media (citrate) on the side of the tube.
2. Using a sterile inoculating loop, obtain a large amount of bacteria and streak the entire slant of the Citrate agar slant.
3. Incubate the inoculated tube in the class test tube rack until next lab session.
AFTER INCUBATION-Observe the color of the Simmons’s citrate slant. Record the reaction and results on the worksheet. Dispose of the citrate slants in the discard rack.
Bright blue = Positive
No color change (stays green) = Negative

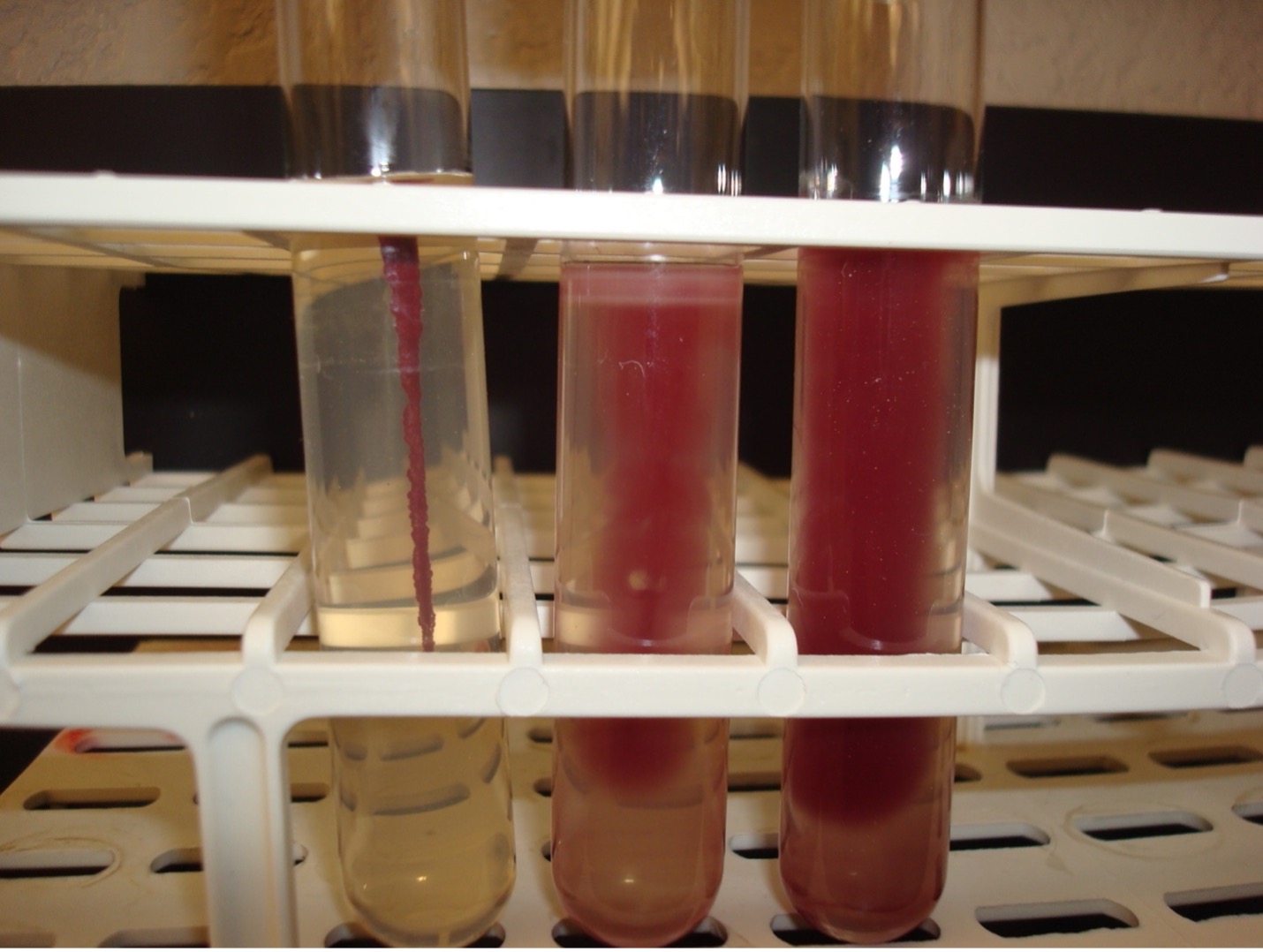
MOTILITY TEST
The motility test detects the ability of bacteria to produce flagella. The media is a semi-solid agar with a colorless dye. As bacteria grow, the dye is reduced by the bacteria and turns red. Bacterial growth is detected by the presence of the red color.
1. Obtain a motility test tube. Use a permanent marker to write the color dot, your name (or initials), and the name of the media (motility) on the side of the tube.
2. Using a sterile inoculating WIRE, obtain a small amount of bacteria. Carefully stab the media about two thirds of the way down. Keep the wire in the same line it entered as you remove it from the media.
3. Incubate the inoculated tube in the class test tube rack until next lab session.
AFTER INCUBATION-Observe the bacterial growth in the media. If the bacteria migrate away from the inoculation stab, the red dye will diffuse in the tube where the bacteria are growing. If the bacteria are not able to migrate, the red dye will be seen only in the stab line where the bacteria are growing. Record the reaction and results on the worksheet. Dispose of the motility agar in the discard rack.
Diffused red color = Positive
Red color only at the stab line = Negative
TEST RESULTS OF ENTEROBACTERIACEAE
| TEST | E. coli | Klebsiella aerogenes | Proteus mirabilis | Klebsiella pneumoniae | Salmonella | Shigella |
| Sucrose fermentation | Negative | Positive | Negative | Positive | Negative | Negative |
| Urease | Negative | Negative | Positive | Positive | Negative | Negative |
| Indole | Positive | Negative | Negative | Negative | Negative | Positive |
| MR Test | Positive | Negative | Positive | Negative | Positive | Positive |
| VP Test | Negative | Positive | Negative | Positive | Negative | Negative |
| Citrate | Negative | Positive | Positive | Positive | Positive | Negative |
| Motility | Positive | Positive | Positive | Negative | Positive | Negative |
pOsT TEST
DISCOVERIES IN MICROBIOLOGY
 DR. BARUJ BENACERRAF
DR. BARUJ BENACERRAF
In the 1940’s, Venezuelan immunologist Dr. Benacerraf discovered the major histocompatibility complex genes which encode cell surface protein molecules critical to the immune system’s distinction between self and non-self. Benacerraf also helped develop our understanding of the phagocytic activity of macrophages, described the functions of IgG subclasses, discovered Fc receptors, demonstrated that T and B cells recognize antigens differently, and observed how B and T cells cooperate with each other to generate an antibody response.

