STAPHYLOCOCCUS
LEARNING OBJECTIVES
Discuss the biochemical tests used to identify Staphylococcus
Differentiate Staphylococcus from other Gram-positive cocci found in clusters on Gram stain
MCCCD OFFICIAL COURSE COMPETENCIES
Describe the modes of bacterial and viral reproduction and proliferation
Utilize aseptic technique for safe handling of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cell
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
MATERIALS
Stock Cultures:
Staphylococcus aureus on TSA slant
Staphylococcus epidermidis on TSA slant
Micrococcus luteus on TSA slant
Micrococcus roseus on TSA slant
Media:
4 glucose fermentation tubes/group
2 Mannitol Salt Agar plates/per group
One tube (1 ml) rabbit plasma/per group
Equipment:
Bottle of hydrogen peroxide
Sterile transfer pipette
2 glass slides/ per person
Inoculating loop/per person
BIOCHEMICAL TESTS ALBUM LINK
Microorganisms metabolize protein, carbohydrates, and lipids to produce energy in much the same way that humans do. Their ability to metabolize these macromolecules is dependent on their ability to produce the specific enzymes that catalyze the biochemical reactions. Since enzymes are proteins, a cell’s genetic code will determine what enzymes an organism can produce. Since the genetic code is not the same in all microorganisms, microorganisms will differ in their ability to produce specific enzymes.
When a microorganism produces an enzyme and a metabolic biochemical reaction occurs, end products will be produced. We can test for the presence of enzymes by studying these biochemical processes and detecting the products of the reactions. Often the products of a biochemical reaction will produce a change in pH of the media in which they occur. If we add a pH indicator to the media, we often can tell if a biochemical reaction occurs by a change in the color of the media. Other times a biochemical reaction will destroy one of the components that makes up the media and the media will physically change in some way. Hemolysis (destruction of red cells) is an example of a change in media composition because of microbial enzymes.
Selective and/or differential media is used to identify microorganisms. Selective media contains substances that allow some microorganisms to grow but prevent the growth of other organisms. Differential media contains substances that help distinguish microorganisms that grow on the media either by changes that occur in the media or by changes that occur in the appearance of the microorganism.
Selective and/or differential media may be used in Petri plates or in tube media (both solid and liquid). Usually, several tests are performed to establish unique patterns of growth and metabolic processes to identify the organism. Commercially prepared media often combine a series of these tests in packaged identification systems to be used for specific groups of organisms. The API 20E system and the Enterotube II system are two examples of these products that are available for purchase.
Gram stains and other differential stains can help us group microorganisms, but we can use selective and differential media based on biochemical testing to identify these organisms according to their genus and species.
Gram-positive cocci are ubiquitous in nature. Their natural habitats in humans and other animals include the skin and mucous membranes in the mouth, nasal passages, throat, anus and vagina. When Gram-positive cocci are isolated from a clinical specimen, the first consideration is to determine if the isolate is a member of the Micrococcaceae (Staphylococcus or Micrococcus) or the Streptococcaceae family (Streptococcus or Enterococcus). The differentiation can be made on the results of the colony appearance, the arrangement of the cells as seen in a Gram stain, and the catalase test reaction.
Colonies of Staphylococcus or Micrococcus are usually larger (2 to 3 mm. after 24 hours of incubation) than Streptococcus or Enterococcus colonies (pinpoint or smaller than 2 mm after 24 hours of incubation). Staphylococcusand Micrococcus colonies are usually convex, opaque and frequently pigmented. Streptococcus and Enterococcuscolonies are often translucent (see-through) or semi-opaque. Staphylococcus and Micrococcus are catalase positive and on Gram-stain are frequently seen in grape-like clusters. Streptococcus and Enterococcus are catalase negative and when stained from a broth, are seen in pairs or chains.
In this exercise, we will learn how to identify four members of the Micrococcaceae family (Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus luteus and Micrococcus roseus). These are normally found on the skin and mucous membranes in the mouth, nasal passages, throat, anus and vagina.
Staphylococcus aureus is usually considered to be pathogenic, but it may be found in the absence of disease. Some people are carriers of this organism, meaning they harbor the pathogen but exhibit no symptoms. One study found as many as 90% of hospital personnel were S. aureus carriers! This poses a risk for hospitalized patients whose immune systems may already be stressed or compromised by their underlying condition. MRSA is especially dangerous because these isolates have become resistant to penicillin derivatives, such as methicillin and oxacillin.
Staphylococcus aureus produces enzymes (hyaluronidase and collagenase) to help it invade and parasitize healthy tissue. Staphylococcus aureus produces coagulase and staphylokinase that hijack the host’s coagulation system. It can cause infections ranging from superficial (boils, impetigo) to systemic infections which involve deeper tissues, such as bone (osteomyelitis), lung (pneumonia), heart valves (endocarditis), brain (meningitis), and blood (toxic shock syndrome). Staphylococcus aureus is the most common etiologic agent of wound infections, including surgical infections. It is a common cause of Healthcare Acquired/Associated Infections (HAI) which are acquired in hospitals and other healthcare facilities. And, many strains produce an exotoxin which causes food poisoning.
Staphylococcus epidermidis is the predominant species of Staphylococcus found on the skin and usually not pathogenic.
Micrococcus luteus and Micrococcus roseus are also considered to be avirulent, though it is important to remember that under the proper circumstances, almost any bacteria can cause disease.
PRE-ASSESSMENT
PROCEDURE
For this exercise: Work in groups of 4. Each person in the group will work with one of color dot cultures and perform the following tests:
CATALASE TEST
This test determines the ability of the bacterium to produce the enzyme catalase. Hydrogen peroxide is toxic to the cell when it accumulates during aerobic respiration. The bacteria must be able to break down H2O2 into H2O and O2 to survive.
This is first test used to differentiate Staphylococcus and Micrococcus from Streptococcus and Enterococcus.
1. Obtain a glass slide and a small bottle of hydrogen peroxide.
2. Using a sterilized inoculating loop, smear a small amount of organism onto the dry slide. Place the slide on a dark background for better observation.
3. Place a drop of hydrogen peroxide on top of the bacterial smear. The immediate appearance of bubbles indicates the organism can produce catalase.
Bubbles = Positive
No bubbles = Negative
4. Record the result on the worksheet
5. Dispose of the slide in the used slide basin at the back of the lab.

SLIDE COAGULASE TEST
This test will determine the ability of the bacteria to produce bound coagulase. This exoenzyme will cause the conversion of fibrinogen in plasma to fibrin and form a clot. The fibrin covers the surface of the bacteria, making it resistant to phagocytosis.
1. Obtain a glass slide, sterile transfer pipette and a tube of rabbit plasma (rabbit plasma is in the short test tube and is clear).
2. Put a drop of rabbit plasma on the slide.
3. Mix a large amount of inoculum into the plasma. Mix the bacteria and plasma for one minute.
4. Place the slide on a dark background for better observation. Obvious clotting of the rabbit plasma indicates the organism produces bound coagulase. Clumps of bacteria from not mixing for one minute do not indicate clotting.
Clotting with a clear background = Positive
*No clotting with a milky background = Negative
*Please note clumps of bacteria from not mixing for one minute do not indicate clotting.

5. Record the result on the worksheet. Optional: If you are unsure of the coagulase test result, inoculate a tube of rabbit plasma and check for a clot in the tube. This is considered a positive reaction for coagulase.
6. Dispose of the slide in the used slide basin at the back of the lab.
PIGMENT PRODUCTION
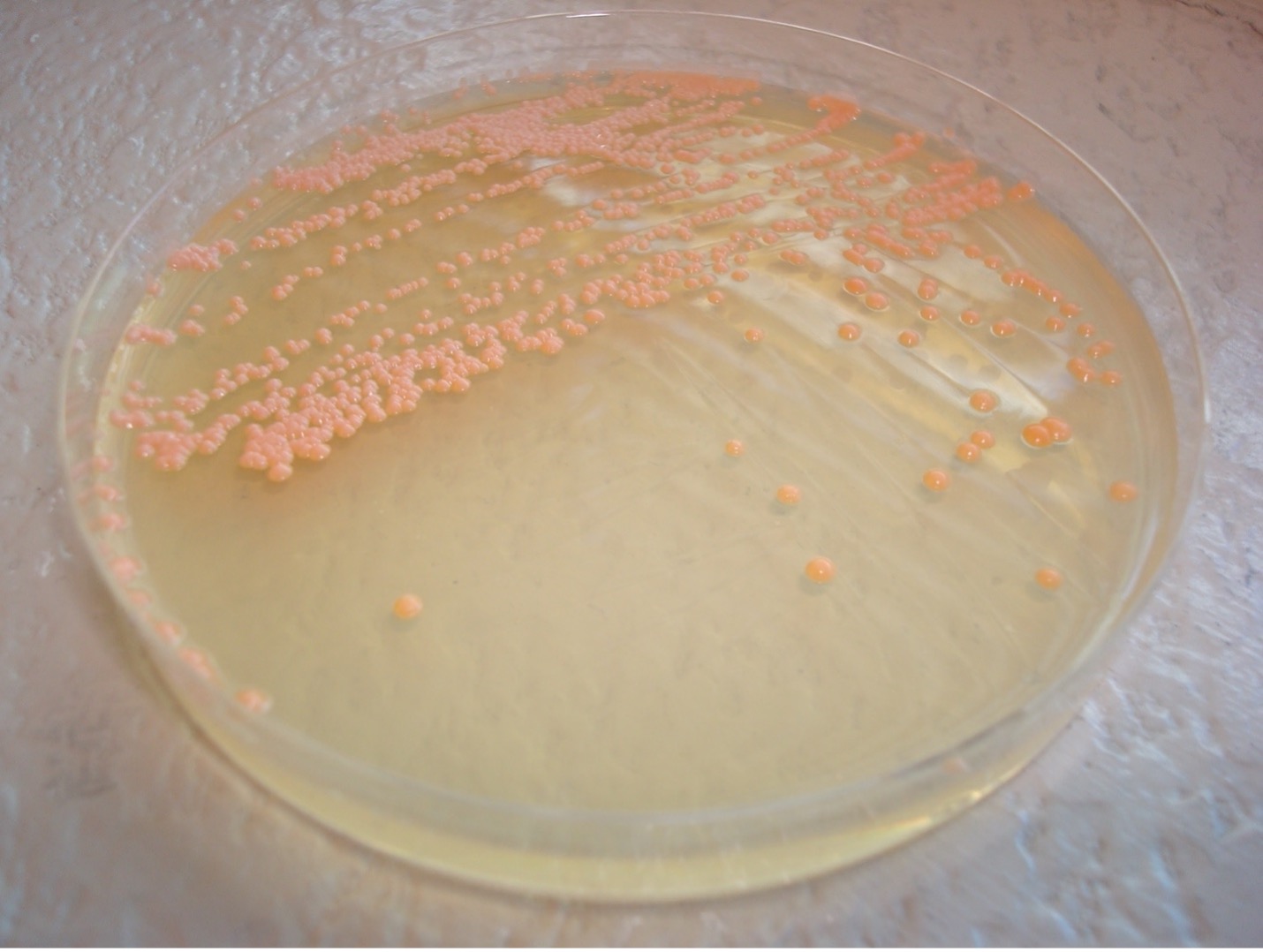
Staphylococcus will produce a white pigment while some species of Micrococcus will produce colorful pigment (rose-coral, yellow) which can serve as an identifying characteristic.
1. Observe the growth on the TSA colored dot slant or your TSA Gram positive stock plate for pigment production.
2. Record the color on the worksheet.
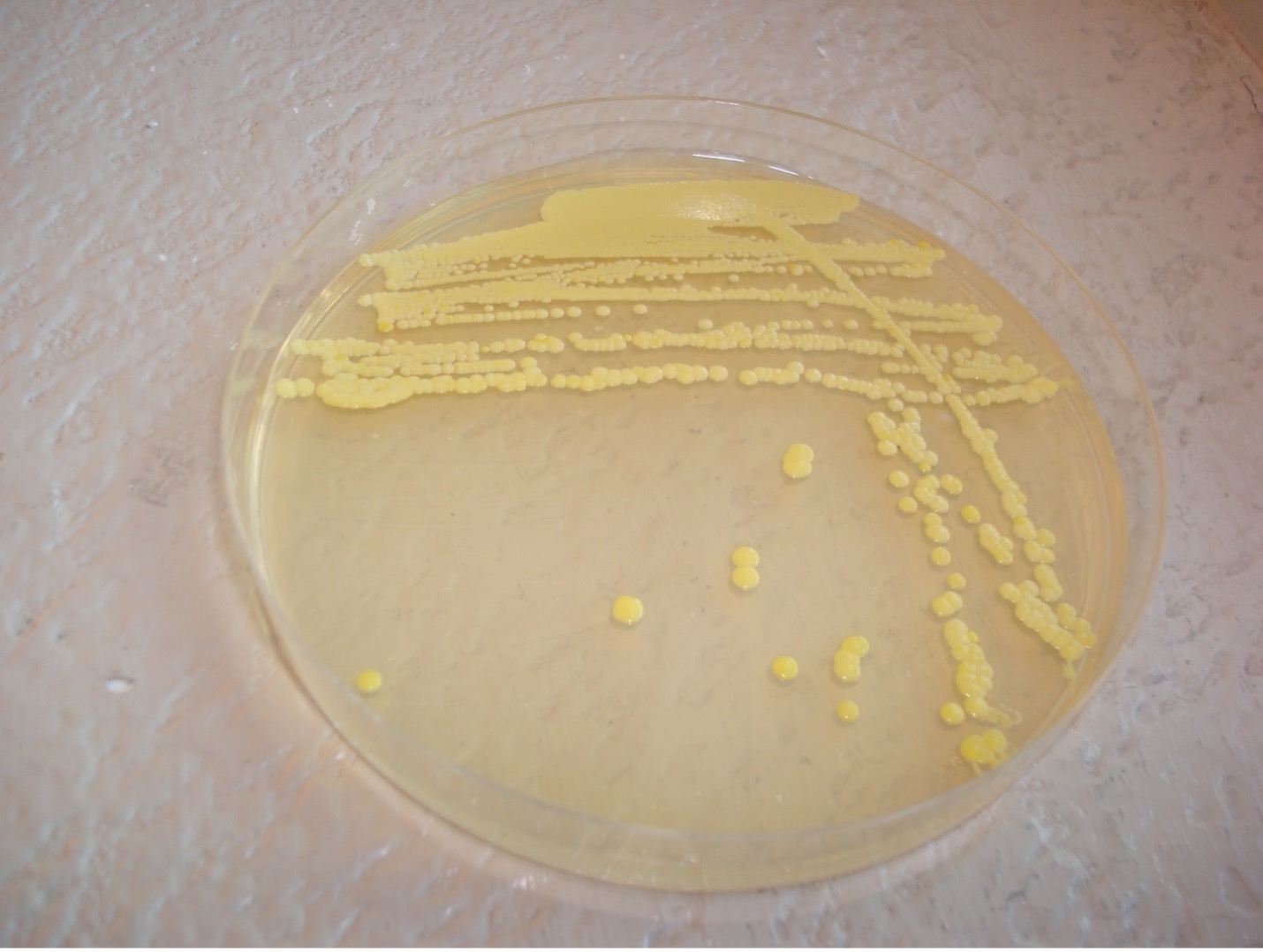
GLUCOSE FERMENTATION BROTH TEST
This test detects the ability of the bacterium to break down glucose to pyruvic acid. If acid is produced, the pH indicator will turn yellow.
1. Obtain a glucose fermentation tube. Make a tape label writing the color dot, your name (or initials), and the name of the media (glucose).
2. Using a sterile inoculating loop, obtain a small amount of inoculum and inoculate the glucose fermentation tube.
3. Incubate the fermentation tube in the class test tube rack until the next lab session.
AFTER INCUBATION-Observe the appearance of the glucose fermentation tube. Record the result on the worksheet.
Yellow broth = Positive test
Red broth = Negative test

Dispose of the glucose fermentation tubes in the discard rack at the back of the lab.
MANNITOL SALT AGAR
This is a selective and differential media which grows and differentiates Staphylococcus species. Since Staphylococcus is salt-tolerant, it will be able to grow in 7.5% salt and Staphylococcus aureus will be differentiated by mannitol fermentation.
1. Each person will use one-half of a mannitol salt agar plate. Label one-half of the agar with your color dot, your name, and the name of the media (MSA).
2. Inoculate your assigned organism on one-half of the agar as shown below.
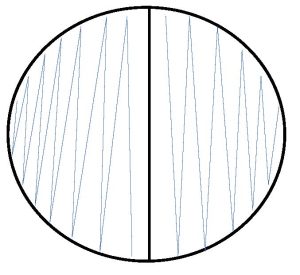
3. Incubate the mannitol agar plate in the class plate tray at 37oC.
AFTER INCUBATION– There are two results to observe. Record results on the worksheet.
Evaluate how the well the organism tolerated the salt in the agar.
Bacteria grew very well = Positive
Bacteria were inhibited from growing = Negative
If the bacteria grew very well on the media, then evaluate the organism’s ability to ferment the mannitol in the agar.
Media turned yellow = Positive = mannitol was fermented (Do not confuse the yellow pigmented colonies of Micrococcus luteus as mannitol fermentation)
Media stayed reddish pink = Negative = mannitol was NOT fermented
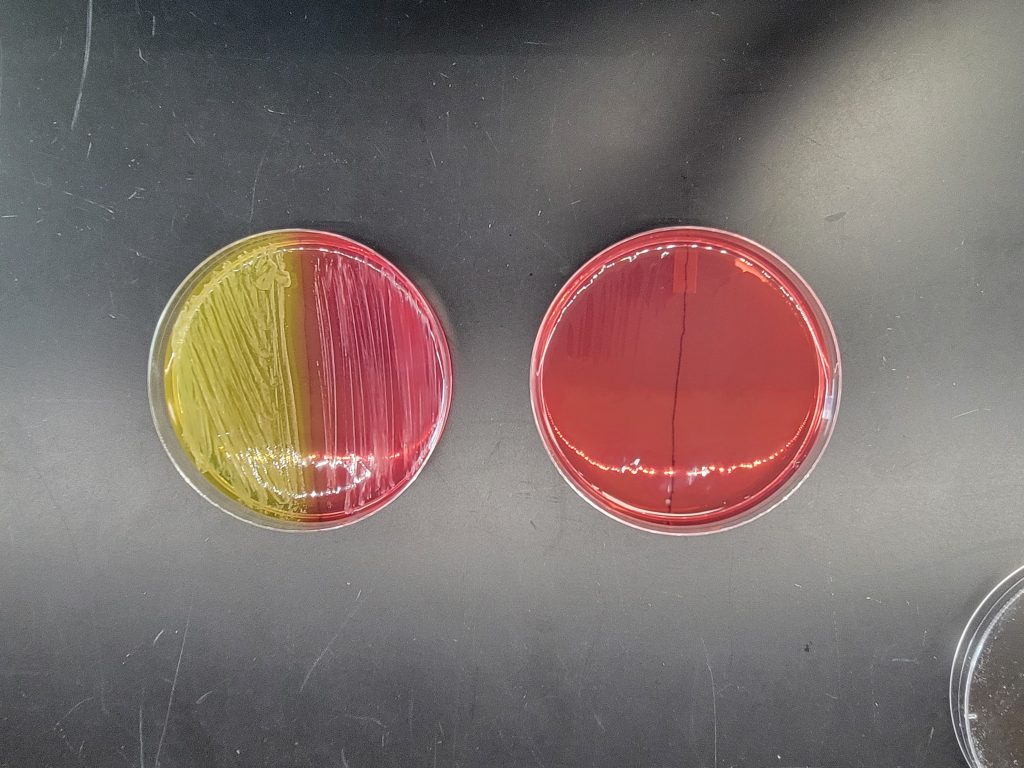
Dispose of the mannitol salt agar plates in the autoclave trash.
TEST RESULTS OF STAPHYLOCOCCUS AND MICROCOCCUS
| TEST | Staphylococcus aureus | Staphylococcus epidermidis | Micrococcus roseus | Micrococcus luteus |
|---|---|---|---|---|
| Catalase | Positive | Positive | Positive | Positive |
| Glucose Fermentation | Positive | Positive | Negative | Negative |
| Coagulase | Positive | Negative | Negative | Negative |
| Salt Tolerance on Mannitol Salt Tolerance | Positive | Positive | Negative | Negative |
| Mannitol Fermentation on Mannitol salt Agar | Positive | Negative | Negative | Negative |
| Pigment Production (colony color) | White | White | Rose-Coral | Yellow |
| Nitrate Reduction (not done in this exercise) | Positive | Positive | Positive | Negative |
POST TEST
DISCOVERIES IN MICROBIOLOGY
 MARY HNATUSKO HUNT
MARY HNATUSKO HUNT
In the 1940’s American bacteriologist Mary Hnatusko Hunt worked at a USDA research lab in Peoria Illinois. She visited the Illinois Fruit and Vegetable Company on Main Street in Peoria. She noticed the owner removing a moldy grapefruit from a display. She asked the owner if he could set aside moldy items for her to pick up on a regular basis, he agreed. At some point, Mary told him it was no longer necessary to save moldy fruit and vegetables for her. Several years later, Mary came to the store to inform the owner that a cantaloupe he provided was the source of penicillin. Mary helped isolate and test many strains of Penicillium.

