ALPHA AND BETA HEMOLYTIC STREPTOCOCCUS
LEARNING OBJECTIVES
Perform biochemical tests used to identify Streptococcus and Enterococcus
Differentiate pathogenic Streptococcus and Enterococcus
MCCCD OFFICIAL COURSE COMPETENCIES
Describe the modes of bacterial and viral reproduction and proliferation
Utilize aseptic technique for safe handling of microorganisms
Apply various laboratory techniques to identify types of microorganisms
Identify structural characteristics of the major groups of microorganisms
Compare and contrast prokaryotic cell and eukaryotic cell
Compare and contrast the physiology and biochemistry of the various groups of microorganisms
MATERIALS
Day 1
Media: per group of 3 students
3 blood agar plate
3 bile esculin slant
Equipment:
3 glass slides
Bottle of hydrogen peroxide
Inoculating loops
Stock Cultures:
TSA Slant culture of Group C Streptococcus
TSA Slant culture of Streptococcus sanguinis
TSA Slant culture of Enterococcus faecalis
Day 2
Media:
Blood agar plate/ 2 students share 1 plate
Optochin disks- on instructor’s desk
Tweezers in alcohol- on instructor’s desk
Lancefield grouping test kits for Grouping Beta hemolytic Streptococcus
Demonstrations:
Blood agar plate of optochin sensitive Streptococcus pneumoniae
Beta, Alpha and Gamma hemolysis on blood agar
BIOCHEMICAL TESTS ALBUM LINK
Organisms in the Streptococcaceae family include Streptococcus and Enterococcus. The colonies of Streptococcus or Enterococcus colonies are smaller than 2 mm. in diameter after 24 hours of incubation and are often translucent (see-through) or semi-transparent. Streptococcus and Enterococcus are Gram-positive cocci in pairs or chains when stained from a broth. They are differentiated from Staphylococcus and Micrococcus by the catalase reaction. The Streptococcaceae cannot produce catalase.
The Streptococcus are initially classified according to their hemolytic properties. After determining the hemolysis, subsequent biochemical testing can narrow down the identification even further.
HEMOLYSIS
Some bacteria produce hemolysins that damage the membrane of red blood cells. This process is called hemolysis. The degree to which the blood cells are hemolyzed is used to distinguish bacteria from one another. There are three types of hemolysis, designated as beta, alpha and gamma.
Beta hemolysis represents a complete lysing of the red blood cells. This is demonstrated by a complete clearing of the red blood cells in the media around a colony leaving only the color of the base (TSA) media.
Alpha hemolysis represents a partial damage of the red blood cells which reduces the hemoglobin to methemoglobin. This partial damage is shown as an olive green to brown discoloration in the media surrounding the bacterial colony on the blood agar.
Gamma hemolysis represents the lack of hemolysis. There is no damage to the red blood cells in the media.
BETA HEMOLYTIC STREPTOCOCCUS – LANCEFIELD GROUPING
In the early 1930’s Rebecca Lancefield developed Lancefield grouping. Lancefield grouping is a serological method for classifying streptococci into one of twenty groups (designated by a letter) based on the presence of polysaccharide and teichoic acid antigens in the bacterial cell wall. In most clinical laboratories only Groups A, B, and C are routinely identified since these groups are responsible for most of human infections. Lancefield grouping is performed using a test kit, which allow rapid detection of streptococcal groups. Although many Lancefield groups include more than one species in each group, Streptococcus pyogenes is the only member of Lancefield’s Group A and Streptococcus agalactiae is the only member of Lancefield’s Group B Streptococcus.
Beta-hemolytic Group A Streptococcus cause ninety percent of acute streptococcal infections in humans. It is the cause of “strep throat” and is particularly worrisome because of the delayed sequelae that may appear. Delayed sequelae are post-infection conditions that may develop days or weeks after the sore throat has healed. These conditions include rheumatic fever and glomerulonephritis. Scarlet fever is also caused by certain exotoxins of Group A Streptococcus. People are usually infected by close contact with another person who is colonized with the bacteria.
Beta-hemolytic Group B Streptococcus has been recognized as potentially dangerous for newborns. It can cause septicemia, meningitis, and pneumonia. The most common mode of acquisition by the neonate is exposure to the microbiome of the maternal genital tract in utero through ruptured membranes or by contamination during passage through the birth canal. In cows, Streptococcus agalactiae causes bovine mastitis.
Beta-hemolytic Group C Streptococcus includes several species (Examples-S. constellatus, S. intermedius, S. anginosus, S. dysgalactiae, and S. equi). Group C streptococci are a common cause of infection in several animal species but are generally considered to be a rare cause of infection in humans.
Group D Streptococcus can demonstrate alpha, beta, or gamma hemolysis and are normal inhabitants of the human intestinal tract. Streptococcus bovis and Streptococcus equinus are two species in Group D that are found in cows and horses but are not usually considered to be pathogenic for those animals.
Group G Streptococcus cause disease in dogs and cats. Group G streptococci are part of the normal microbiome of the pharynx, gastrointestinal tract, genital tract, and skin. However, case reports have indicated Group G in complicated infections, including cellulitis, osteomyelitis, septic arthritis, meningitis, endocarditis, and bacteremia.
ALPHA HEMOLYTIC STREPTOCOCCUS
Streptococcus pneumoniae is an alpha-hemolytic, lancet shaped diplococcus that is the most frequent cause of middle ear infections (otitis media) and bacteremia in infants and children. It is also the main cause of community-acquired pneumonia in the United States. It is frequently isolated from the upper respiratory tract of 30-70% of healthy individuals. The pathogenic strains of Streptococcus pneumoniae produce a capsule that inhibits phagocytosis. Although over 90 serotypes exist, only a minority of serotypes cause diseases. These serotypes are included in a polyvalent vaccine.
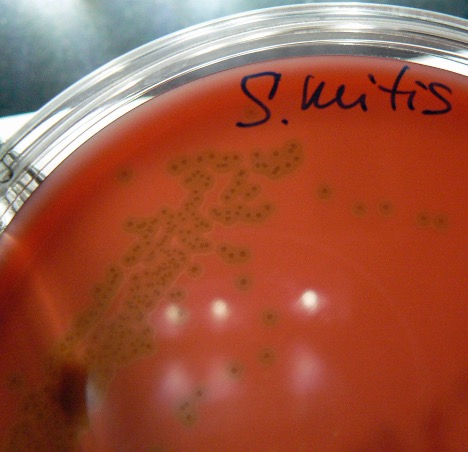
Other alpha hemolytic streptococci are often referred to as “viridans” streptococci because of the greenish discoloration (alpha hemolysis) seen in the media. Streptococcus sanguinis is an example of a viridans streptococcus. It is considered part of the normal microbiome in the mouth and forms oral biofilms known as dental plaque. It also is the most common viridans streptococcus associated with bacterial endocarditis (infection of the heart valve or endocardium). Streptococcus mutans is another viridans streptococcus associated with dental caries and plaque.
ENTEROCOCCUS
The enterococci are among the most ubiquitous bacteria known. They are found in human and animal gastrointestinal tracts and the environment. There are many different species of enterococci, but only a few have the potential to cause infections in humans. More than 95% of infections due to enterococci are caused by just two species, Enterococcus faecium and Enterococcus faecalis. These bacteria are not very invasive but are significant opportunists causing urinary tract infections, pelvic abscesses, peritonitis and wound infections in humans. Recently many strains have acquired resistance to Vancomycin and have been named “VRE” for Vancomycin Resistant Enterococcus.
PRE-ASSESSMENT
PROCEDURE
For this exercise: Each person in the group will work with a colored dot culture and perform the following tests.
CATALASE TEST
This is first test used to differentiate Staphylococcus and Micrococcus from Streptococcus and Enterococcus.
This test determines the ability of the bacterium to produce the enzyme catalase. Hydrogen peroxide is toxic to the cell when it accumulates during aerobic respiration. The bacteria must be able to break down H2O2 into H2O and O2 to survive.
1. Obtain a glass slide and a small bottle of hydrogen peroxide.
2. Using a sterilized inoculating loop, smear a small amount of organism onto the dry slide. Place the slide on a dark background for better observation.
3. Place a drop of hydrogen peroxide on top of the bacterial smear.
4. The immediate appearance of bubbles indicates the organism can produce catalase. Record the result on the worksheet.
Bubbles = Positive
No bubbles = Negative

BILE ESCULIN HYDROLYSIS TEST
This test will detect the ability of the bacterium to hydrolyze bile esculin. The Enterococcus and Group D Streptococcus can be differentiated from other streptococci by this test.
1. Obtain one bile esculin agar slant. Using a sterilized inoculating loop, use a large amount of bacteria to inoculate the entire bile esculin agar slant. Label with your name (or initials), the name of the media (bile esculin) and your assigned culture.
2. Incubate the slant in the class test tube rack until next lab session.
AFTER INCUBATION-Determine if the bacteria could hydrolyze the esculin in the agar. Record the result on the worksheet.
Very dark brown/black media = Positive
No color change in the media = Negative
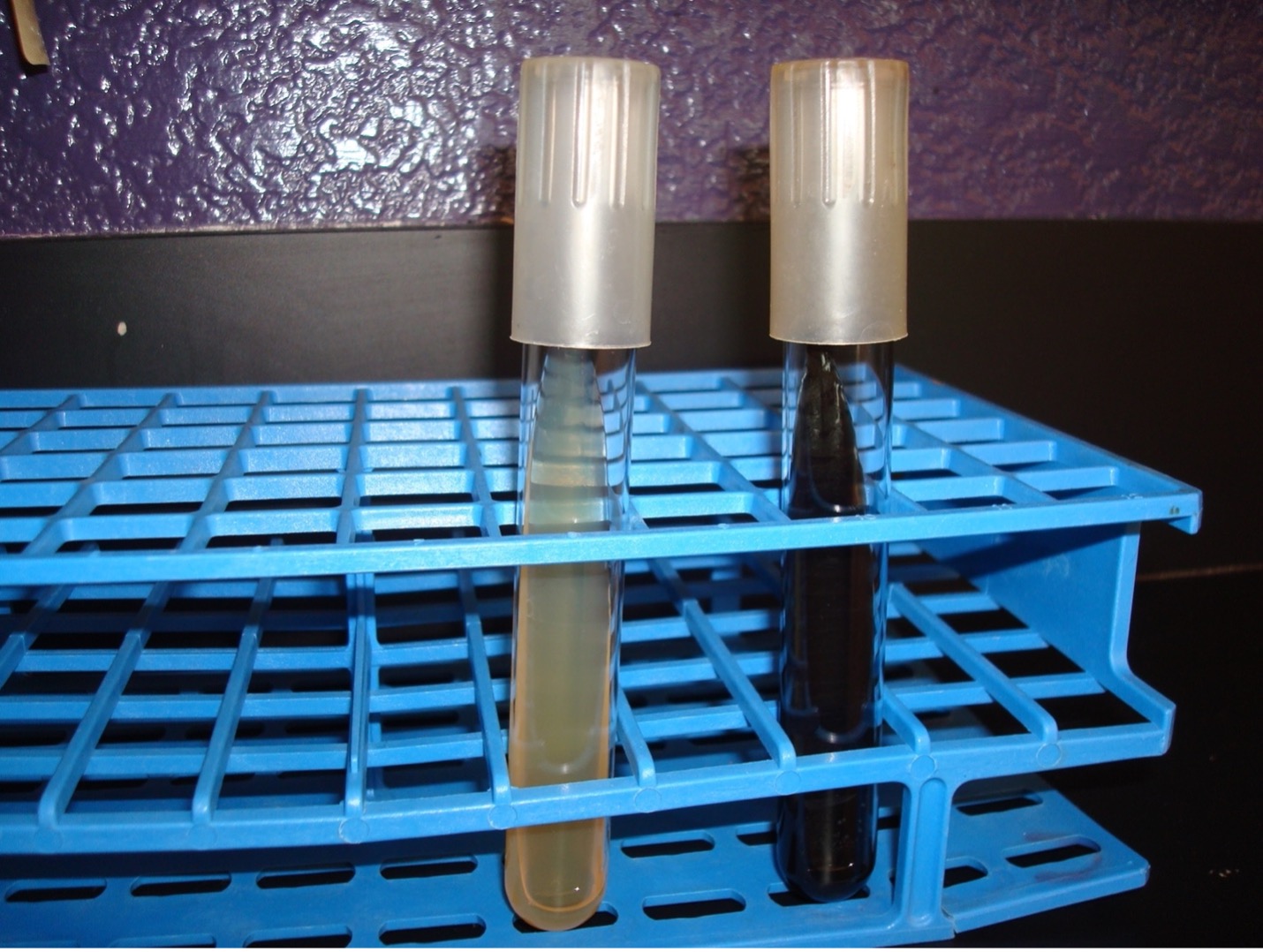
Dispose of the Bile Esculin slants in the discard rack at the back of the lab.
HEMOLYSIS ON BLOOD AGAR TEST
Traditional method streak plate
1. Obtain one blood agar plate. Label the bottom of the plate with your assigned culture, name of the media (blood agar), and your name. Draw a T on the bottom of the plate to divide the plate into three sections.
2. Sterilize your inoculating loop in the Bacticinerator and allow it to cool. Remove the cap from your assigned culture with your little finger. Use a sterile inoculating loop to obtain the inoculum from your assigned culture. Recap your assigned culture and place it in a test tube rack.
3. Using your non-dominant hand, open the lid of the plate just enough to fit the loop in.
4. cInoculate the first section of the plate by touching the loop down on the surface of the agar and drawing a zig-zag streak to fill that first area of the plate. Replace the Petri plate lid.
5. Sterilize your inoculating loop in the Bacticinerator and allow it to cool.
6. Open the lid of the plate just enough to fit the loop in. Place the loop in the center of the first section and gently drag the loop one time into the second section. Lightly drag the tip of the loop from side to side in a back-and-forth motion to spread the inoculum to fill the second section. Replace the Petri plate lid.
7. Sterilize the loop in the Bacticinerator and allow it to cool.
8. Open the lid of the plate just enough to fit the loop in. Place the loop in the center of the second section and gently drag the loop one time into the third section. Lightly drag the tip of the loop from side to side in a back-and-forth motion to spread the inoculum to fill the third section. Replace the Petri Plate lid.
9. Sterilize the loop in the Bacticinerator and allow it to cool.
10. Stab the loop 2–3 times into the agar in the first section of the streak plate. Stabbing into the media will enhance the appearance of hemolysis.
11. Incubate the inverted blood agar plate in the candle jar at 37oC until the next lab session. Most Streptococci grow best in a microaerophilic environment.
AFTER INCUBATION-Evaluate the type of hemolysis produced by the organisms. Hold the plate so that light is shining through the back of the agar. Determine the extent of damage to the blood in the agar surrounding the bacterial colonies. If necessary, use the inoculating loop to scrape away a few colonies to see the media underneath the colonies. Record the result on the worksheet.
IF THE ORGANISM DEMONSTRATES:
Alpha or gamma hemolysis: perform the Optochin sensitivity test
Beta hemolysis: perform Lancefield grouping
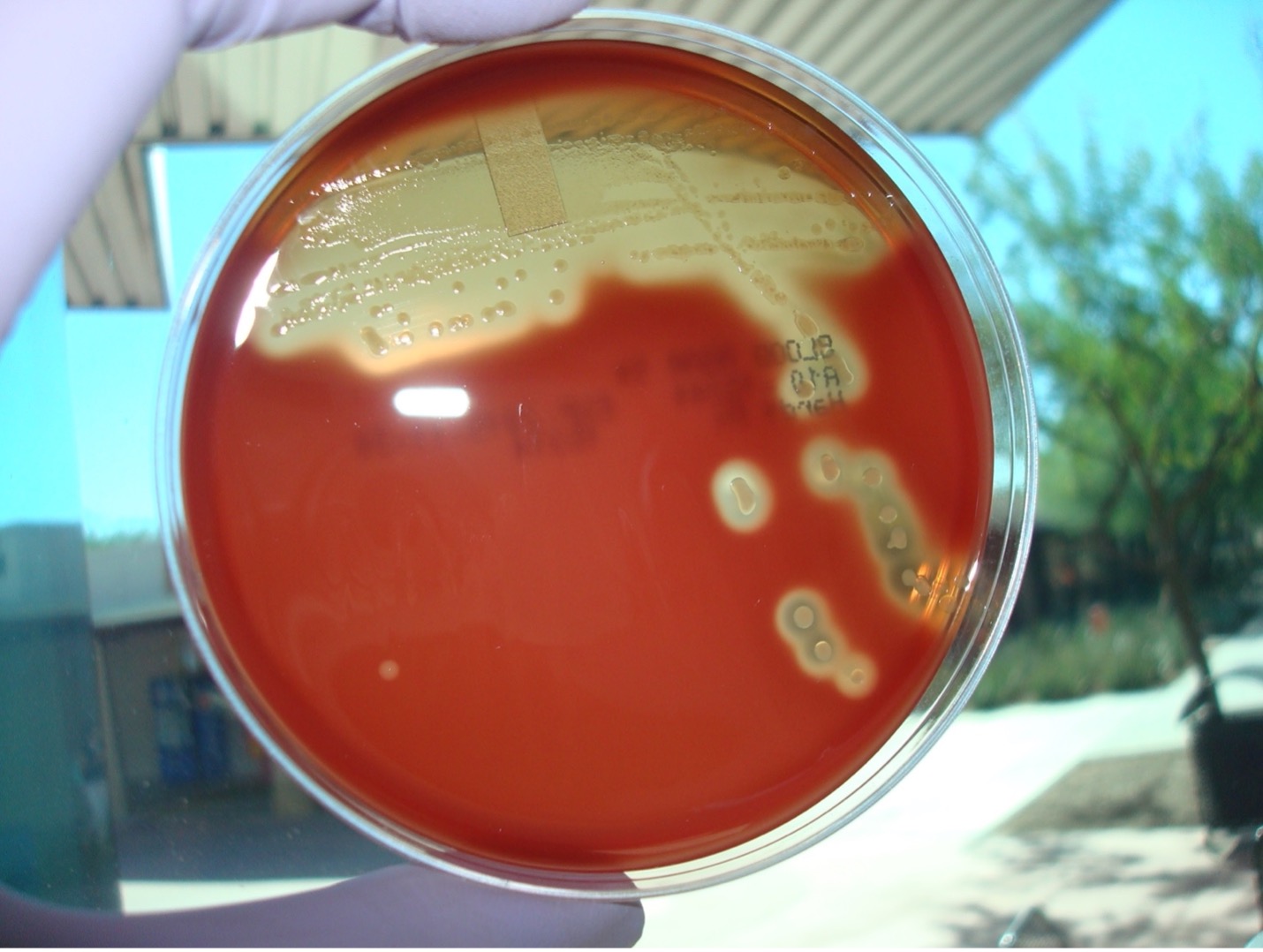
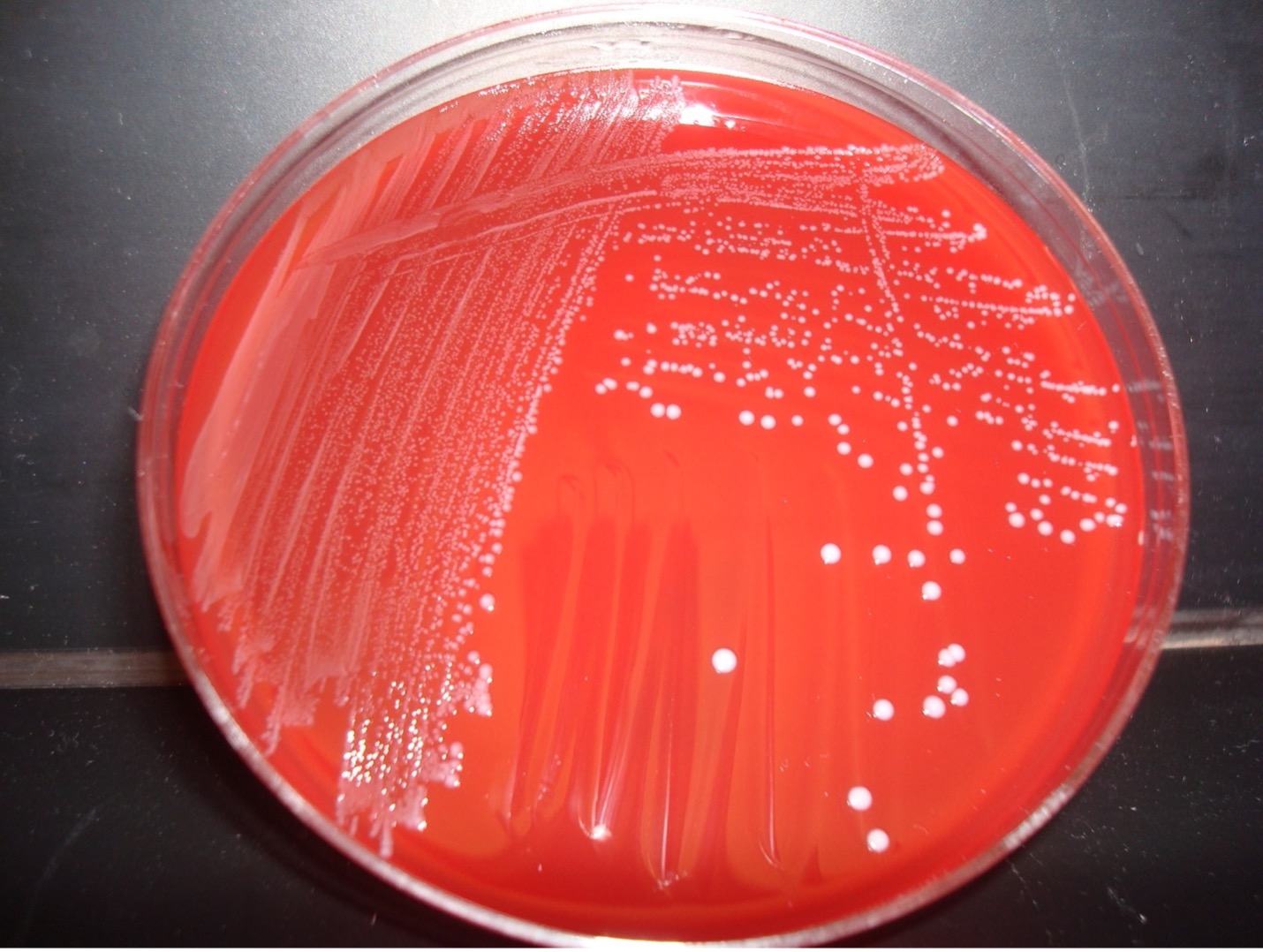
alternative method streak plate
1. Obtain one blood agar plate. Label the bottom of the plate with your assigned culture, name of the media (blood agar), and your name. Draw a T on the bottom of the plate to divide the plate into three sections.
2. Sterilize your inoculating loop in the Bacticinerator and allow it to cool. Remove the cap from your assigned culture with your little finger. Use a sterile inoculating loop to obtain the inoculum from your assigned culture. Recap your assigned culture and place it in a test tube rack.
3. Using your non-dominant hand, open the lid of the plate just enough to fit the loop in.
4. Inoculate the first section of the plate by touching the loop down on the surface of the agar and drawing a zig-zag streak to fill that first area of the plate. Replace the Petri plate lid.
5. Sterilize your inoculating loop in the Bacticinerator and allow it to cool.
6. Open the lid of the plate just enough to fit the loop in. Inoculate the second section of the plate by touching the loop down in the middle of the first section of the plate and draw a line toward the second section. Continue to draw parallel lines to fill the second section. Replace the Petri plate lid.
7. Sterilize your inoculating loop in the Bacticinerator and allow it to cool.
8. Open the lid of the plate just enough to fit the loop in. Inoculate the third section of the plate by touching the loop down by touching down in the middle of the second section of the plate and draw a line toward the third section. Continue to draw parallel lines to fill the third section. Replace the Petri plate lid.
9. Sterilize your inoculating loop in the Bacticinerator and allow it to cool.
10. Stab the loop 2–3 times into the agar in the first section of the streak plate. Stabbing into the media will enhance the appearance of hemolysis.
11. Incubate the inverted blood agar plate in the candle jar at 37oC until the next lab session. Most Streptococci grow best in a microaerophilic environment.
AFTER INCUBATION-Evaluate the type of hemolysis produced by the organisms. Hold the plate so that light is shining through the back of the agar. Determine the extent of damage to the blood in the agar surrounding the bacterial colonies. If necessary, use the inoculating loop to scrape away a few colonies to see the media underneath the colonies. Record the result on the worksheet.
IF THE ORGANISM DEMONSTRATES:
Alpha or gamma hemolysis: perform the Optochin sensitivity test
Beta hemolysis: perform Lancefield grouping



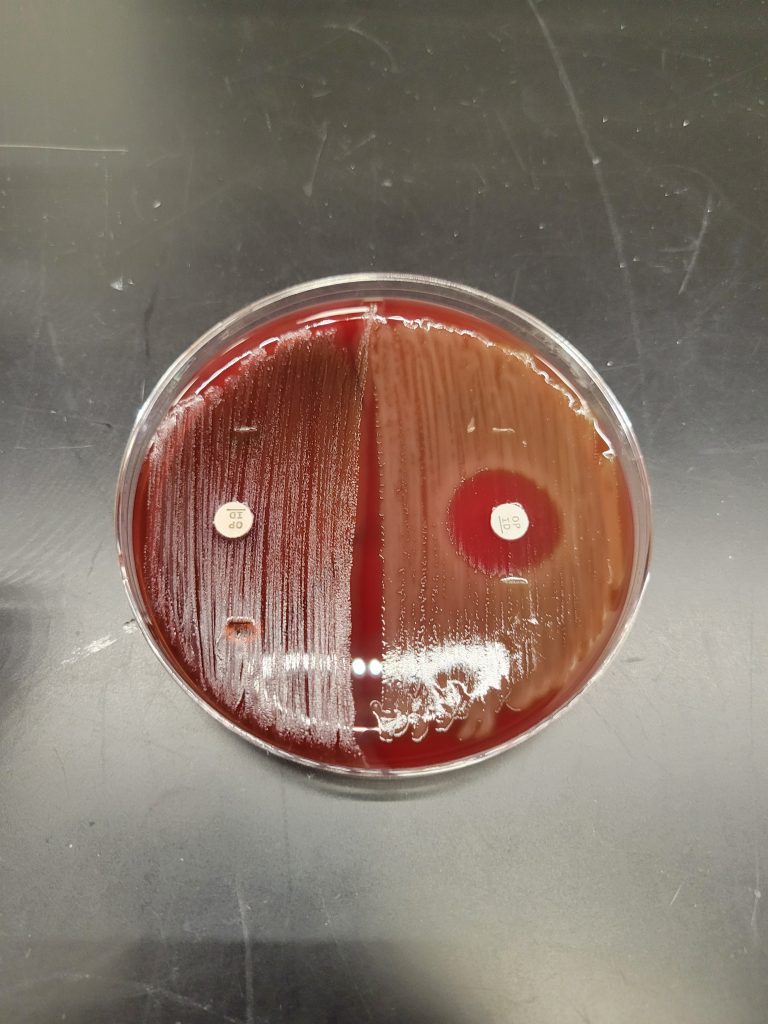
FOR ALPHA OR GAMMA HEMOLYTIC STREPTOCOCCUS: OPTOCHIN SENSITIVITY TEST
This test differentiates Streptococcus pneumoniae from other alpha hemolytic streptococci. S. pneumoniae is sensitive to the chemical optochin (ethylhdrocupreine hydrochloride) and will be inhibited from growing around the optochin disk.
1. Label half of a blood agar plate with your name. Another student will also use this plate to inoculate their unknown.
2. Use a sterile inoculating loop and pick a few isolated colonies of your unknown bacteria growing on the agar plate. Heavily streak the organism onto the half of the labeled blood agar plate creating a confluent lawn of growth.
3. Use the dispenser to dispense an optochin disk on the media in the middle of the inoculated area. Take the tweezers out of the alcohol and let them dry completely. Use the tweezers to gently press the disk to ensure it is in contact with the plate. DO NOT press the disk into the agar.
4. Incubate the blood agar in the Candle jar until next lab session.
5. Dispose of the blood agar plate you used to determine hemolysis in the autoclave trash.
AFTER INCUBATION-Determine if the bacteria are sensitive or resistant to Optochin. Record the results on the worksheet. Dispose of the optochin sensitivity test plate in the autoclave trash.
FOR BETA HEMOLYTIC STREPTOCOCCUS: LANCEFIELD GROUPING
Principle of the Procedure:
A lancefield grouping test kit will allow you to determine if the beta hemolytic streptococci is Lancefield group A or Lancefield group C. You will extract the group antigen from the streptococci cell wall. The group antigen will then be mixed antibodies to group A and C. The Group A and C antibodies are on blue latex particles. Group A antibodies will bind to the group A antigen. Group C antibodies will bind to the group C antigen. When antibody binds to antigen clumping occurs, you will see distinct blue clumps with a clear background. When antibody does not bind to antigen you will not see distinct blue clumps. If the beta hemolytic streptococci is group A you will see clumping in the Group A labeled circle due to A antibodies binding to the extracted group A antigen. If the beta hemolytic streptococci is group C you will see clumping in the Group C labeled circle due to C antibodies binding to the extracted group C antigen.
Procedure:
1. Obtain one empty test tube to test your bacteria.
2. Find Reagent 1 in the kit, Add one drop of Extraction Reagent 1 to the test tube.
3. Using a sterile inoculating loop, take a loopful (yes a full loopful) of your unknown bacteria growing on the agar plate and suspend it in Extraction Reagent 1.
4. Find Reagent 2 in the kit. Add one drop of Extraction Reagent 2 to the test tube. Mix by tapping the test tube with your finger for 10 seconds.
5. Find Reagent 3 in the kit. Add five drops of Extraction Reagent 3 to the test tube. Mix the contents of the tube by tapping the tube with your finger for 10 seconds. You have now successfully extracted the Group A or C antigen from the cell wall of the bacteria.
6. Find a test card in the kit. Label one circle on the test card “Group A” and another circle “Group C”. Find the Antibody A Reagent and Antibody C Reagent in the kit. Shake the Antibody A and Antibody C reagents gently a few times.
7. Add one drop of Antibody A reagent to the circle on the test card you labeled “Group A”. Add one drop of the Antibody C reagent to the circle on the test card you labeled “Group C”.
8. Using a Pasteur pipette, place one drop of the bacteria extract from the test tube into each circle. Do not allow the pipette tip to touch the drop of antibody reagent already on the test card.
9. Find a wooden stick in the kit. Mix the drop of antibody and the bacteria extract with the side of a wooden stick to fill the complete circle. A new stick should be used for each reagent.
10. Gently hand rock the entire card, allowing the mixture to flow slowly within the ring area.
11. After rocking the card for one minute, observe for clumping of the blue particles. Record the result on the worksheet. Dispose of the Lancefield grouping test and the blood agar plate in the autoclave trash.
Positive results:
Clumping of the blue particles with one of the antibodies indicates if the beta hemolytic streptococci is Group A or Group C.
Negative Results:
No visible clumping indicates a negative reaction for the Lancefield group.
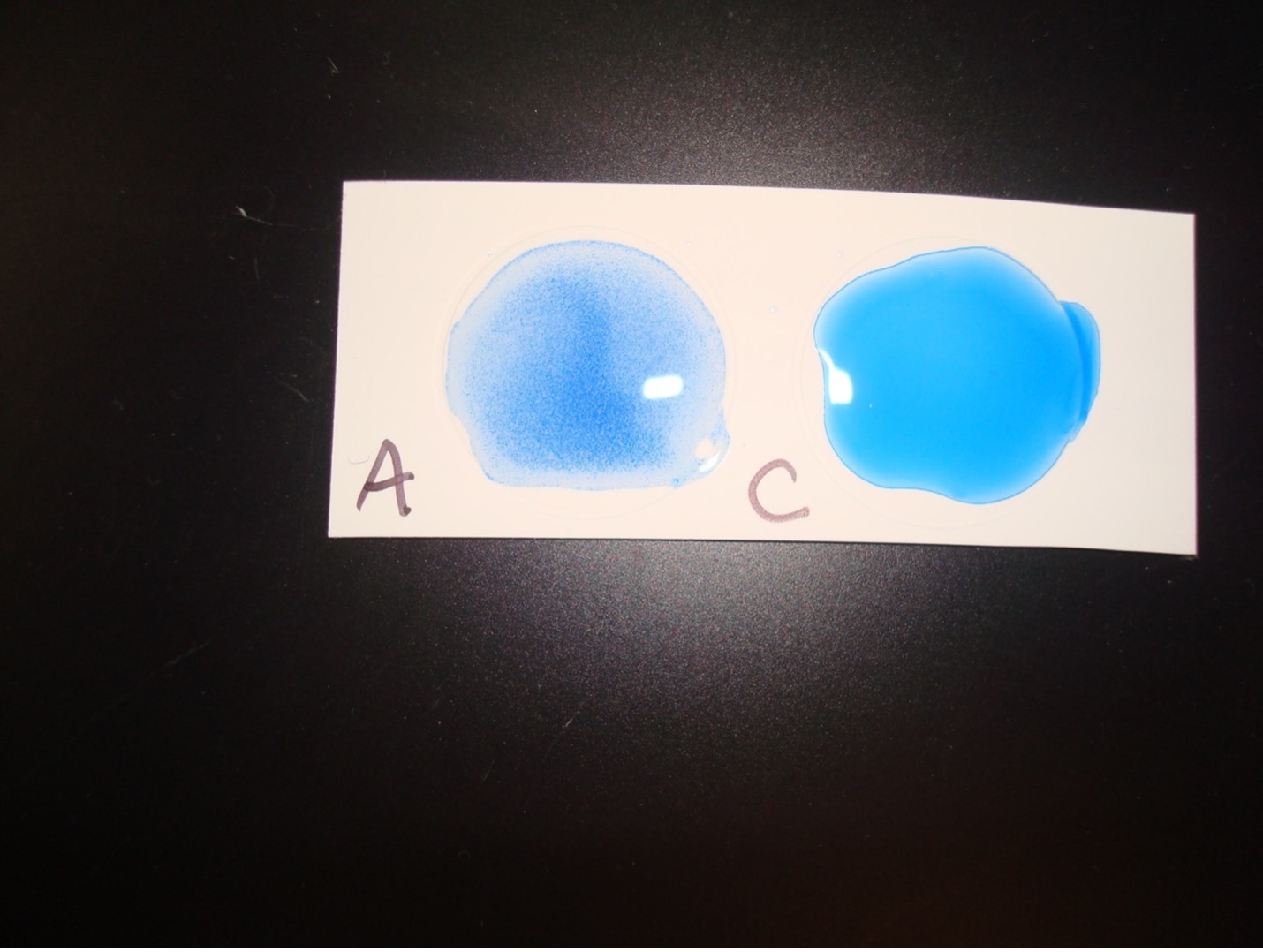
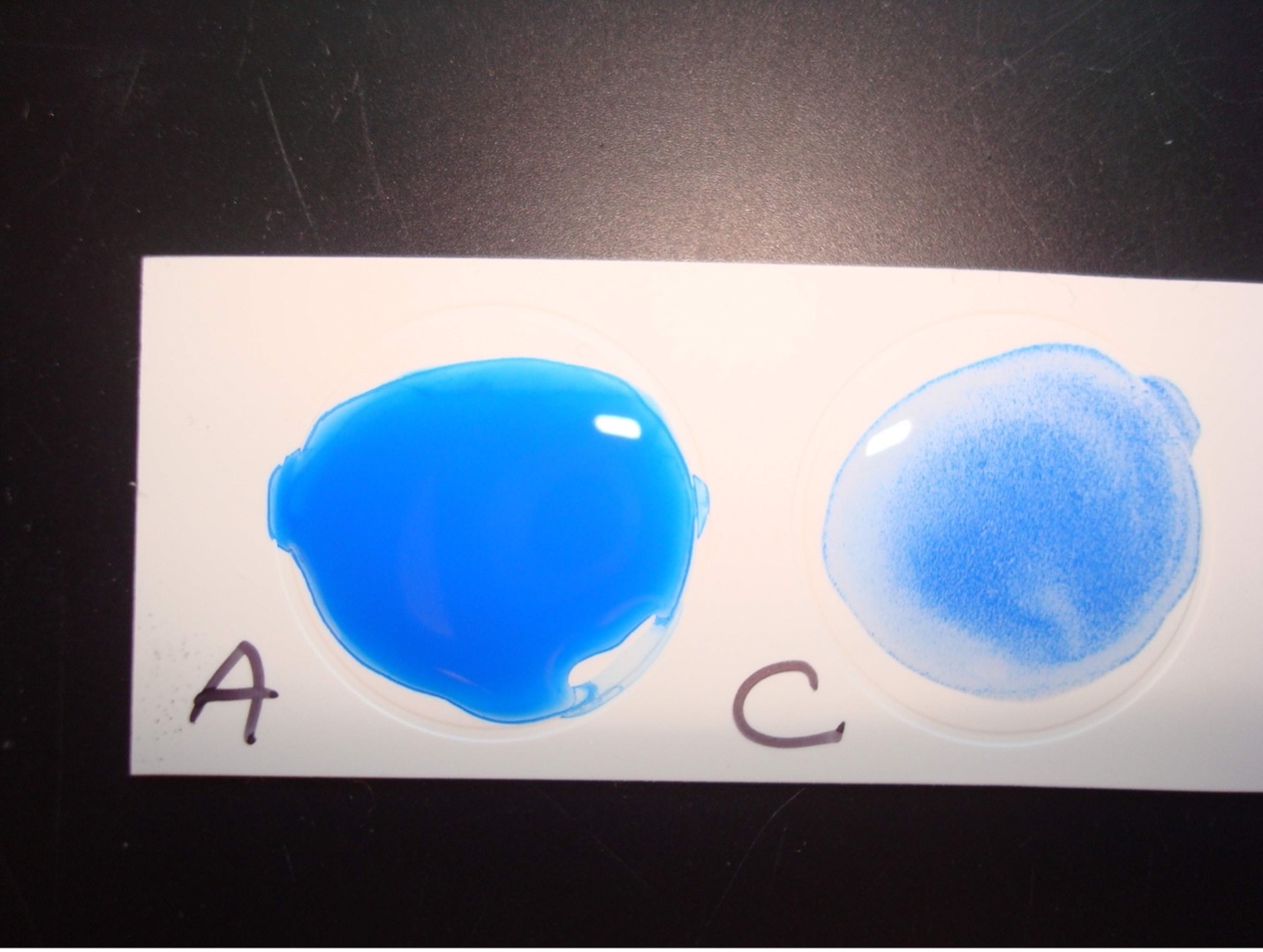
TEST RESULTS OF ALPHA, BETA, AND GAMMA HEMOLYTIC STREPTOCOCCUS AND ENTEROCOCCUS
| TEST | Streptococcus sanguinis | Streptococcus pneumoniae | Streptococcus pyogenes | Group C Streptococcus |
Enterococcus faecalis |
| Catalase | Negative | Negative | Negative | Negative | Negative |
| Bile Esculin | Negative | Negative | Negative | Negative | Positive |
| Hemolysis | Alpha | Alpha | Beta | Beta | *Variable |
| Optochin sensitivity | Resistant (Negative) |
Sensitive (Positive) |
|||
| Latex Grouping | Clumping with Group A antibodies | Clumping with Group C antibodies |
NOTE: “Variable” test result means the result is not consistent. Some strains of the species will exhibit gamma hemolysis whereas other strains will produce alpha or beta hemolysis.
POST TEST
DISCOVERIES IN MICROBIOLOGY
 SARA LITTLE TURNBULL
SARA LITTLE TURNBULL
American product designer Sara Little Turnbull consulted with companies including General Mills, Coca-Cola, and Revlon. Her work with 3M would change medical care. 3M hired her to explore uses (including gift ribbons and bra cups) for a new melded polymer fiber material. At the time, she was taking care of sick family members and spending a lot of time in hospitals. She wondered if the material might block disease particles. In 1961, 3M started selling a mask based on Sara’s design. The mask blocked dust and airborne toxins, but did not block pathogens. Her mask design was used by others to create N95 masks, which are critical to stopping disease transmission.

